| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Glutamate receptor ionotropic, NMDA 1 |
|---|
| Ligand | BDBM50005076 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEBML_141849 |
|---|
| IC50 | 11300±n/a nM |
|---|
| Citation |  Leeson, PD; Carling, RW; Moore, KW; Moseley, AM; Smith, JD; Stevenson, G; Chan, T; Baker, R; Foster, AC; Grimwood, S 4-Amido-2-carboxytetrahydroquinolines. Structure-activity relationships for antagonism at the glycine site of the NMDA receptor. J Med Chem35:1954-68 (1992) [PubMed] Leeson, PD; Carling, RW; Moore, KW; Moseley, AM; Smith, JD; Stevenson, G; Chan, T; Baker, R; Foster, AC; Grimwood, S 4-Amido-2-carboxytetrahydroquinolines. Structure-activity relationships for antagonism at the glycine site of the NMDA receptor. J Med Chem35:1954-68 (1992) [PubMed] |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Glutamate receptor ionotropic, NMDA 1 |
|---|
| Name: | Glutamate receptor ionotropic, NMDA 1 |
|---|
| Synonyms: | Glutamate (NMDA) receptor subunit zeta 1 | Glutamate [NMDA] receptor subunit zeta-1 | Glutamate-NMDA-Channel | Glutamate-NMDA-MK801 | Glutamate-NMDA-Polyamine | Grin1 | NMDA | NMDZ1_RAT | Nmdar1 | phencyclidine |
|---|
| Type: | Enzyme Catalytic Domain |
|---|
| Mol. Mass.: | 105533.40 |
|---|
| Organism: | RAT |
|---|
| Description: | P35439 |
|---|
| Residue: | 938 |
|---|
| Sequence: | MSTMHLLTFALLFSCSFARAACDPKIVNIGAVLSTRKHEQMFREAVNQANKRHGSWKIQL
NATSVTHKPNAIQMALSVCEDLISSQVYAILVSHPPTPNDHFTPTPVSYTAGFYRIPVLG
LTTRMSIYSDKSIHLSFLRTVPPYSHQSSVWFEMMRVYNWNHIILLVSDDHEGRAAQKRL
ETLLEERESKAEKVLQFDPGTKNVTALLMEARELEARVIILSASEDDAATVYRAAAMLNM
TGSGYVWLVGEREISGNALRYAPDGIIGLQLINGKNESAHISDAVGVVAQAVHELLEKEN
ITDPPRGCVGNTNIWKTGPLFKRVLMSSKYADGVTGRVEFNEDGDRKFANYSIMNLQNRK
LVQVGIYNGTHVIPNDRKIIWPGGETEKPRGYQMSTRLKIVTIHQEPFVYVKPTMSDGTC
KEEFTVNGDPVKKVICTGPNDTSPGSPRHTVPQCCYGFCIDLLIKLARTMNFTYEVHLVA
DGKFGTQERVNNSNKKEWNGMMGELLSGQADMIVAPLTINNERAQYIEFSKPFKYQGLTI
LVKKEIPRSTLDSFMQPFQSTLWLLVGLSVHVVAVMLYLLDRFSPFGRFKVNSEEEEEDA
LTLSSAMWFSWGVLLNSGIGEGAPRSFSARILGMVWAGFAMIIVASYTANLAAFLVLDRP
EERITGINDPRLRNPSDKFIYATVKQSSVDIYFRRQVELSTMYRHMEKHNYESAAEAIQA
VRDNKLHAFIWDSAVLEFEASQKCDLVTTGELFFRSGFGIGMRKDSPWKQNVSLSILKSH
ENGFMEDLDKTWVRYQECDSRSNAPATLTFENMAGVFMLVAGGIVAGIFLIFIEIAYKRH
KDARRKQMQLAFAAVNVWRKNLQDRKSGRAEPDPKKKATFRAITSTLASSFKRRRSSKDT
STGGGRGALQNQKDTVLPRRAIEREEGQLQLCSRHRES
|
|
|
|---|
| BDBM50005076 |
|---|
| n/a |
|---|
| Name | BDBM50005076 |
|---|
| Synonyms: | 4-Amino-5,7-dichloro-1,2,3,4-tetrahydro-quinoline-2-carboxylic acid | CHEMBL62250 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C10H10Cl2N2O2 |
|---|
| Mol. Mass. | 261.105 |
|---|
| SMILES | N[C@H]1C[C@@H](Nc2cc(Cl)cc(Cl)c12)C(O)=O |
|---|
| Structure | 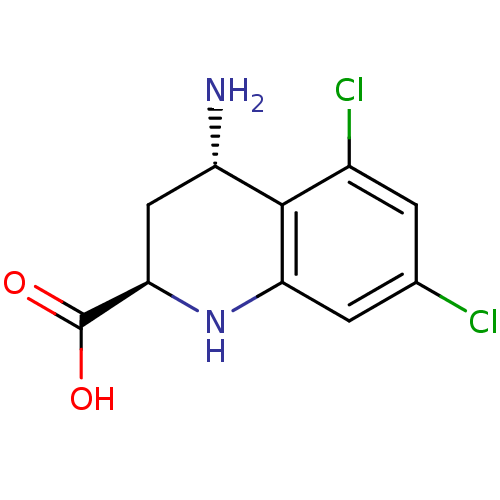
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Leeson, PD; Carling, RW; Moore, KW; Moseley, AM; Smith, JD; Stevenson, G; Chan, T; Baker, R; Foster, AC; Grimwood, S 4-Amido-2-carboxytetrahydroquinolines. Structure-activity relationships for antagonism at the glycine site of the NMDA receptor. J Med Chem35:1954-68 (1992) [PubMed]
Leeson, PD; Carling, RW; Moore, KW; Moseley, AM; Smith, JD; Stevenson, G; Chan, T; Baker, R; Foster, AC; Grimwood, S 4-Amido-2-carboxytetrahydroquinolines. Structure-activity relationships for antagonism at the glycine site of the NMDA receptor. J Med Chem35:1954-68 (1992) [PubMed]