| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Cytochrome P450 2A6 |
|---|
| Ligand | BDBM50366394 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_1735364 (CHEMBL4150900) |
|---|
| IC50 | 400±n/a nM |
|---|
| Citation |  Denton, TT; Srivastava, P; Xia, Z; Chen, G; Watson, CJW; Wynd, A; Lazarus, P Identification of the 4-Position of 3-Alkynyl and 3-Heteroaromatic Substituted Pyridine Methanamines as a Key Modification Site Eliciting Increased Potency and Enhanced Selectivity for Cytochrome P-450 2A6 Inhibition. J Med Chem61:7065-7086 (2018) [PubMed] Article Denton, TT; Srivastava, P; Xia, Z; Chen, G; Watson, CJW; Wynd, A; Lazarus, P Identification of the 4-Position of 3-Alkynyl and 3-Heteroaromatic Substituted Pyridine Methanamines as a Key Modification Site Eliciting Increased Potency and Enhanced Selectivity for Cytochrome P-450 2A6 Inhibition. J Med Chem61:7065-7086 (2018) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Cytochrome P450 2A6 |
|---|
| Name: | Cytochrome P450 2A6 |
|---|
| Synonyms: | 1,4-cineole 2-exo-monooxygenase | 1.14.13.- | CP2A6_HUMAN | CYP2A3 | CYP2A6 | CYPIIA6 | Coumarin 7-hydroxylase | Cytochrome P450 2A6 | Cytochrome P450 IIA3 | Cytochrome P450(I) |
|---|
| Type: | Protein |
|---|
| Mol. Mass.: | 56514.34 |
|---|
| Organism: | Homo sapiens (Human) |
|---|
| Description: | P11509 |
|---|
| Residue: | 494 |
|---|
| Sequence: | MLASGMLLVALLVCLTVMVLMSVWQQRKSKGKLPPGPTPLPFIGNYLQLNTEQMYNSLMK
ISERYGPVFTIHLGPRRVVVLCGHDAVREALVDQAEEFSGRGEQATFDWVFKGYGVVFSN
GERAKQLRRFSIATLRDFGVGKRGIEERIQEEAGFLIDALRGTGGANIDPTFFLSRTVSN
VISSIVFGDRFDYKDKEFLSLLRMMLGIFQFTSTSTGQLYEMFSSVMKHLPGPQQQAFQL
LQGLEDFIAKKVEHNQRTLDPNSPRDFIDSFLIRMQEEEKNPNTEFYLKNLVMTTLNLFI
GGTETVSTTLRYGFLLLMKHPEVEAKVHEEIDRVIGKNRQPKFEDRAKMPYMEAVIHEIQ
RFGDVIPMSLARRVKKDTKFRDFFLPKGTEVYPMLGSVLRDPSFFSNPQDFNPQHFLNEK
GQFKKSDAFVPFSIGKRNCFGEGLARMELFLFFTTVMQNFRLKSSQSPKDIDVSPKHVGF
ATIPRNYTMSFLPR
|
|
|
|---|
| BDBM50366394 |
|---|
| n/a |
|---|
| Name | BDBM50366394 |
|---|
| Synonyms: | CHEMBL4159065 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C16H16Cl2N2O |
|---|
| Mol. Mass. | 323.217 |
|---|
| SMILES | Cl.Cl.NCc1ccc(o1)-c1cnccc1-c1ccccc1 |
|---|
| Structure | 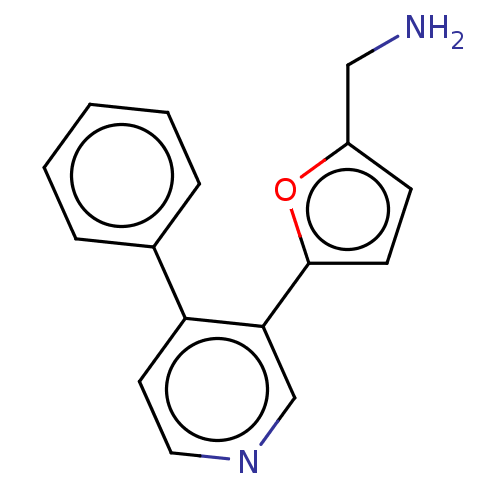
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Denton, TT; Srivastava, P; Xia, Z; Chen, G; Watson, CJW; Wynd, A; Lazarus, P Identification of the 4-Position of 3-Alkynyl and 3-Heteroaromatic Substituted Pyridine Methanamines as a Key Modification Site Eliciting Increased Potency and Enhanced Selectivity for Cytochrome P-450 2A6 Inhibition. J Med Chem61:7065-7086 (2018) [PubMed] Article
Denton, TT; Srivastava, P; Xia, Z; Chen, G; Watson, CJW; Wynd, A; Lazarus, P Identification of the 4-Position of 3-Alkynyl and 3-Heteroaromatic Substituted Pyridine Methanamines as a Key Modification Site Eliciting Increased Potency and Enhanced Selectivity for Cytochrome P-450 2A6 Inhibition. J Med Chem61:7065-7086 (2018) [PubMed] Article