| Reaction Details |
|---|
 | Report a problem with these data |
| Target | G-protein coupled estrogen receptor 1 |
|---|
| Ligand | BDBM50459750 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_1767035 (CHEMBL4202282) |
|---|
| EC50 | 480±n/a nM |
|---|
| Citation |  O'Dea, A; Sondergard, C; Sweeney, P; Arnatt, CK A Series of Indole-Thiazole Derivatives Act as GPER Agonists and Inhibit Breast Cancer Cell Growth. ACS Med Chem Lett9:901-906 (2018) [PubMed] Article O'Dea, A; Sondergard, C; Sweeney, P; Arnatt, CK A Series of Indole-Thiazole Derivatives Act as GPER Agonists and Inhibit Breast Cancer Cell Growth. ACS Med Chem Lett9:901-906 (2018) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| G-protein coupled estrogen receptor 1 |
|---|
| Name: | G-protein coupled estrogen receptor 1 |
|---|
| Synonyms: | CEPR | CMKRL2 | DRY12 | G-protein coupled estrogen receptor 1 | G-protein-coupled receptor (GPR30 ) | GPER | GPER1 | GPER1_HUMAN | GPR30 |
|---|
| Type: | PROTEIN |
|---|
| Mol. Mass.: | 42259.21 |
|---|
| Organism: | Homo sapiens (Human) |
|---|
| Description: | ChEMBL_982507 |
|---|
| Residue: | 375 |
|---|
| Sequence: | MDVTSQARGVGLEMYPGTAQPAAPNTTSPELNLSHPLLGTALANGTGELSEHQQYVIGLF
LSCLYTIFLFPIGFVGNILILVVNISFREKMTIPDLYFINLAVADLILVADSLIEVFNLH
ERYYDIAVLCTFMSLFLQVNMYSSVFFLTWMSFDRYIALARAMRCSLFRTKHHARLSCGL
IWMASVSATLVPFTAVHLQHTDEACFCFADVREVQWLEVTLGFIVPFAIIGLCYSLIVRV
LVRAHRHRGLRPRRQKALRMILAVVLVFFVCWLPENVFISVHLLQRTQPGAAPCKQSFRH
AHPLTGHIVNLAAFSNSCLNPLIYSFLGETFRDKLRLYIEQKTNLPALNRFCHAALKAVI
PDSTEQSDVRFSSAV
|
|
|
|---|
| BDBM50459750 |
|---|
| n/a |
|---|
| Name | BDBM50459750 |
|---|
| Synonyms: | CHEMBL4204670 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C15H13BrClN3OS |
|---|
| Mol. Mass. | 398.705 |
|---|
| SMILES | CCc1nc(NC(=O)c2[nH]c3ccc(Br)cc3c2Cl)sc1C |
|---|
| Structure | 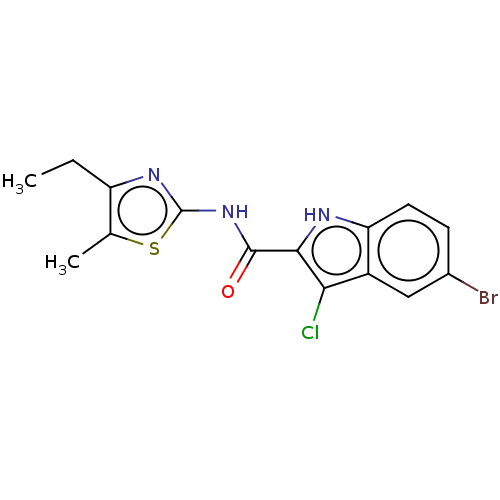
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 O'Dea, A; Sondergard, C; Sweeney, P; Arnatt, CK A Series of Indole-Thiazole Derivatives Act as GPER Agonists and Inhibit Breast Cancer Cell Growth. ACS Med Chem Lett9:901-906 (2018) [PubMed] Article
O'Dea, A; Sondergard, C; Sweeney, P; Arnatt, CK A Series of Indole-Thiazole Derivatives Act as GPER Agonists and Inhibit Breast Cancer Cell Growth. ACS Med Chem Lett9:901-906 (2018) [PubMed] Article