| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Reverse transcriptase |
|---|
| Ligand | BDBM50138406 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_1767095 (CHEMBL4202342) |
|---|
| IC50 | 15±n/a nM |
|---|
| Citation |  Alexandre, FR; Rahali, R; Rahali, H; Guillon, S; Convard, T; Fillgrove, K; Lai, MT; Meillon, JC; Xu, M; Small, J; Dousson, CB; Raheem, IT Synthesis and Antiviral Evaluation of Carbocyclic Nucleoside Analogs of Nucleoside Reverse Transcriptase Translocation Inhibitor MK-8591 (4'-Ethynyl-2-fluoro-2'-deoxyadenosine). J Med Chem61:9218-9228 (2018) [PubMed] Article Alexandre, FR; Rahali, R; Rahali, H; Guillon, S; Convard, T; Fillgrove, K; Lai, MT; Meillon, JC; Xu, M; Small, J; Dousson, CB; Raheem, IT Synthesis and Antiviral Evaluation of Carbocyclic Nucleoside Analogs of Nucleoside Reverse Transcriptase Translocation Inhibitor MK-8591 (4'-Ethynyl-2-fluoro-2'-deoxyadenosine). J Med Chem61:9218-9228 (2018) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Reverse transcriptase |
|---|
| Name: | Reverse transcriptase |
|---|
| Synonyms: | n/a |
|---|
| Type: | Protein |
|---|
| Mol. Mass.: | 29598.37 |
|---|
| Organism: | Human immunodeficiency virus 1 |
|---|
| Description: | Q9WKE8 |
|---|
| Residue: | 254 |
|---|
| Sequence: | PISPITVPVKLKPGMDGPKVKQWPLTEEKIKALTEICTEMEKEGKIEKIGPENPYNTPVF
AIKKKDSTKWRKVVDFRELNKRTQDFWEVQLGIPHPAGLKKKKSVTVLDVGDAYFSVPLD
KDFRKYTAFTIPSINNETPGIRYQYNVLPQGWKGSPAIFQSSMTKILEPFRKQNPDIVIY
QYMDDLYVGSDLEIEQHRAKIEELRQHLLRWGFTTPDKKHQKEPPFLWMGYELHPDKWTV
QPIVLPEKDSWTVN
|
|
|
|---|
| BDBM50138406 |
|---|
| n/a |
|---|
| Name | BDBM50138406 |
|---|
| Synonyms: | 3TC Triphosphate | CHEMBL1230 | LAMIVUDINE | Lamivudine triphosphate | [(2R,5S)-5-(4-amino-2-oxopyrimidin-1(2H)-yl)-1,3-oxathiolan-2-yl]methyl tetrahydrogen triphosphate | [[[5-(4-amino-2-oxo-1H-pyrimidin-1-yl)-1,3-oxathiolan-2-yl]methoxy-hydroxy-phosphoryl]oxy-hydroxy-phosphoryl]oxyphosphonic acid |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C8H14N3O12P3S |
|---|
| Mol. Mass. | 469.196 |
|---|
| SMILES | Nc1ccn([C@@H]2CS[C@H](COP(O)(=O)OP(O)(=O)OP(O)(O)=O)O2)c(=O)n1 |r| |
|---|
| Structure | 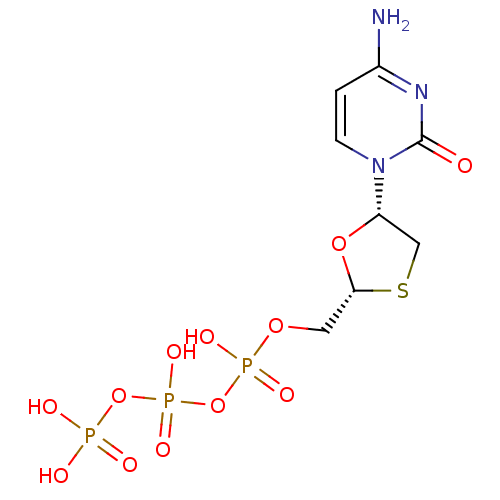
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Alexandre, FR; Rahali, R; Rahali, H; Guillon, S; Convard, T; Fillgrove, K; Lai, MT; Meillon, JC; Xu, M; Small, J; Dousson, CB; Raheem, IT Synthesis and Antiviral Evaluation of Carbocyclic Nucleoside Analogs of Nucleoside Reverse Transcriptase Translocation Inhibitor MK-8591 (4'-Ethynyl-2-fluoro-2'-deoxyadenosine). J Med Chem61:9218-9228 (2018) [PubMed] Article
Alexandre, FR; Rahali, R; Rahali, H; Guillon, S; Convard, T; Fillgrove, K; Lai, MT; Meillon, JC; Xu, M; Small, J; Dousson, CB; Raheem, IT Synthesis and Antiviral Evaluation of Carbocyclic Nucleoside Analogs of Nucleoside Reverse Transcriptase Translocation Inhibitor MK-8591 (4'-Ethynyl-2-fluoro-2'-deoxyadenosine). J Med Chem61:9218-9228 (2018) [PubMed] Article