| Reaction Details |
|---|
 | Report a problem with these data |
| Target | ATP-citrate synthase |
|---|
| Ligand | BDBM50066694 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_1803487 (CHEMBL4275779) |
|---|
| Ki | 220±n/a nM |
|---|
| Citation |  Granchi, C ATP citrate lyase (ACLY) inhibitors: An anti-cancer strategy at the crossroads of glucose and lipid metabolism. Eur J Med Chem157:1276-1291 (2018) [PubMed] Article Granchi, C ATP citrate lyase (ACLY) inhibitors: An anti-cancer strategy at the crossroads of glucose and lipid metabolism. Eur J Med Chem157:1276-1291 (2018) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| ATP-citrate synthase |
|---|
| Name: | ATP-citrate synthase |
|---|
| Synonyms: | ACLY | ACLY_HUMAN |
|---|
| Type: | PROTEIN |
|---|
| Mol. Mass.: | 120848.43 |
|---|
| Organism: | Homo sapiens (Human) |
|---|
| Description: | ChEMBL_455196 |
|---|
| Residue: | 1101 |
|---|
| Sequence: | MSAKAISEQTGKELLYKFICTTSAIQNRFKYARVTPDTDWARLLQDHPWLLSQNLVVKPD
QLIKRRGKLGLVGVNLTLDGVKSWLKPRLGQEATVGKATGFLKNFLIEPFVPHSQAEEFY
VCIYATREGDYVLFHHEGGVDVGDVDAKAQKLLVGVDEKLNPEDIKKHLLVHAPEDKKEI
LASFISGLFNFYEDLYFTYLEINPLVVTKDGVYVLDLAAKVDATADYICKVKWGDIEFPP
PFGREAYPEEAYIADLDAKSGASLKLTLLNPKGRIWTMVAGGGASVVYSDTICDLGGVNE
LANYGEYSGAPSEQQTYDYAKTILSLMTREKHPDGKILIIGGSIANFTNVAATFKGIVRA
IRDYQGPLKEHEVTIFVRRGGPNYQEGLRVMGEVGKTTGIPIHVFGTETHMTAIVGMALG
HRPIPNQPPTAAHTANFLLNASGSTSTPAPSRTASFSESRADEVAPAKKAKPAMPQDSVP
SPRSLQGKSTTLFSRHTKAIVWGMQTRAVQGMLDFDYVCSRDEPSVAAMVYPFTGDHKQK
FYWGHKEILIPVFKNMADAMRKHPEVDVLINFASLRSAYDSTMETMNYAQIRTIAIIAEG
IPEALTRKLIKKADQKGVTIIGPATVGGIKPGCFKIGNTGGMLDNILASKLYRPGSVAYV
SRSGGMSNELNNIISRTTDGVYEGVAIGGDRYPGSTFMDHVLRYQDTPGVKMIVVLGEIG
GTEEYKICRGIKEGRLTKPIVCWCIGTCATMFSSEVQFGHAGACANQASETAVAKNQALK
EAGVFVPRSFDELGEIIQSVYEDLVANGVIVPAQEVPPPTVPMDYSWARELGLIRKPASF
MTSICDERGQELIYAGMPITEVFKEEMGIGGVLGLLWFQKRLPKYSCQFIEMCLMVTADH
GPAVSGAHNTIICARAGKDLVSSLTSGLLTIGDRFGGALDAAAKMFSKAFDSGIIPMEFV
NKMKKEGKLIMGIGHRVKSINNPDMRVQILKDYVRQHFPATPLLDYALEVEKITTSKKPN
LILNVDGLIGVAFVDMLRNCGSFTREEADEYIDIGALNGIFVLGRSMGFIGHYLDQKRLK
QGLYRHPWDDISYVLPEHMSM
|
|
|
|---|
| BDBM50066694 |
|---|
| n/a |
|---|
| Name | BDBM50066694 |
|---|
| Synonyms: | (S)-2-[(R)-7-(3-Chloro-9H-fluoren-9-yl)-2-hydroxy-heptyl]-2-hydroxy-succinic acid | CHEMBL120302 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C24H27ClO6 |
|---|
| Mol. Mass. | 446.921 |
|---|
| SMILES | O[C@H](CCCCCC1c2ccccc2-c2cc(Cl)ccc12)C[C@](O)(CC(O)=O)C(O)=O |
|---|
| Structure | 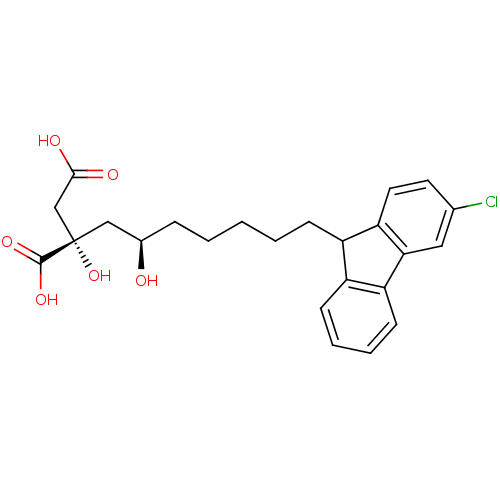
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Granchi, C ATP citrate lyase (ACLY) inhibitors: An anti-cancer strategy at the crossroads of glucose and lipid metabolism. Eur J Med Chem157:1276-1291 (2018) [PubMed] Article
Granchi, C ATP citrate lyase (ACLY) inhibitors: An anti-cancer strategy at the crossroads of glucose and lipid metabolism. Eur J Med Chem157:1276-1291 (2018) [PubMed] Article