| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Alpha-1B adrenergic receptor |
|---|
| Ligand | BDBM50067212 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_32289 (CHEMBL646236) |
|---|
| EC50 | 10965±n/a nM |
|---|
| Citation |  Meyer, MD; Altenbach, RJ; Hancock, AA; Buckner, SA; Knepper, SM; Kerwin, JF Synthesis and in vitro characterization of N-[5-(4,5-dihydro-1H-imidazol-2-yl)-2-hydroxy-5,6,7,8- tetrahydronaphthalen-1-yl]methanesulfonamide and its enantiomers: a novel selective alpha 1A receptor agonist. J Med Chem39:4116-9 (1996) [PubMed] Article Meyer, MD; Altenbach, RJ; Hancock, AA; Buckner, SA; Knepper, SM; Kerwin, JF Synthesis and in vitro characterization of N-[5-(4,5-dihydro-1H-imidazol-2-yl)-2-hydroxy-5,6,7,8- tetrahydronaphthalen-1-yl]methanesulfonamide and its enantiomers: a novel selective alpha 1A receptor agonist. J Med Chem39:4116-9 (1996) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Alpha-1B adrenergic receptor |
|---|
| Name: | Alpha-1B adrenergic receptor |
|---|
| Synonyms: | ADA1B_RAT | Adra1b | Alpha 1B-adrenoceptor | Alpha 1B-adrenoreceptor | Alpha adrenergic receptor 1A and 1B | Alpha-1 Adrenergic Receptor | Alpha-1Adrenoceptor | Alpha-1B adrenergic receptor | Alpha-1B adrenoreceptor | adrenergic Alpha1B |
|---|
| Type: | G Protein-Coupled Receptor (GPCR) |
|---|
| Mol. Mass.: | 56606.71 |
|---|
| Organism: | Rattus norvegicus (rat) |
|---|
| Description: | Receptor binding assays were performed using rat cortical membranes. |
|---|
| Residue: | 515 |
|---|
| Sequence: | MNPDLDTGHNTSAPAHWGELKDDNFTGPNQTSSNSTLPQLDVTRAISVGLVLGAFILFAI
VGNILVILSVACNRHLRTPTNYFIVNLAIADLLLSFTVLPFSATLEVLGYWVLGRIFCDI
WAAVDVLCCTASILSLCAISIDRYIGVRYSLQYPTLVTRRKAILALLSVWVLSTVISIGP
LLGWKEPAPNDDKECGVTEEPFYALFSSLGSFYIPLAVILVMYCRVYIVAKRTTKNLEAG
VMKEMSNSKELTLRIHSKNFHEDTLSSTKAKGHNPRSSIAVKLFKFSREKKAAKTLGIVV
GMFILCWLPFFIALPLGSLFSTLKPPDAVFKVVFWLGYFNSCLNPIIYPCSSKEFKRAFM
RILGCQCRGGRRRRRRRRLGACAYTYRPWTRGGSLERSQSRKDSLDDSGSCMSGTQRTLP
SASPSPGYLGRGTQPPVELCAFPEWKPGALLSLPEPPGRRGRLDSGPLFTFKLLGDPESP
GTEGDTSNGGCDTTTDLANGQPGFKSNMPLAPGHF
|
|
|
|---|
| BDBM50067212 |
|---|
| n/a |
|---|
| Name | BDBM50067212 |
|---|
| Synonyms: | CHEBI:8093 | Cyclomydril | Duo-Medihaler | Phenylephrine |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C9H13NO2 |
|---|
| Mol. Mass. | 167.205 |
|---|
| SMILES | CNC[C@H](O)c1cccc(O)c1 |r| |
|---|
| Structure | 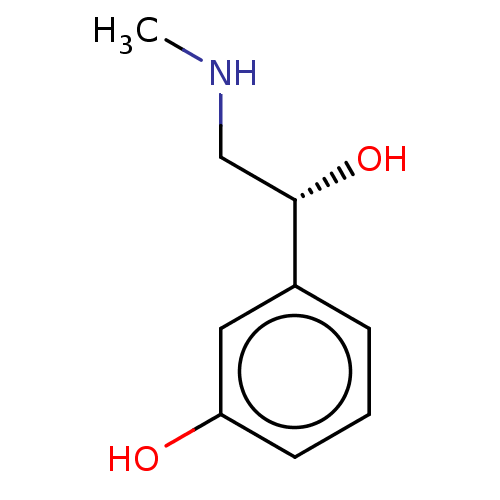
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Meyer, MD; Altenbach, RJ; Hancock, AA; Buckner, SA; Knepper, SM; Kerwin, JF Synthesis and in vitro characterization of N-[5-(4,5-dihydro-1H-imidazol-2-yl)-2-hydroxy-5,6,7,8- tetrahydronaphthalen-1-yl]methanesulfonamide and its enantiomers: a novel selective alpha 1A receptor agonist. J Med Chem39:4116-9 (1996) [PubMed] Article
Meyer, MD; Altenbach, RJ; Hancock, AA; Buckner, SA; Knepper, SM; Kerwin, JF Synthesis and in vitro characterization of N-[5-(4,5-dihydro-1H-imidazol-2-yl)-2-hydroxy-5,6,7,8- tetrahydronaphthalen-1-yl]methanesulfonamide and its enantiomers: a novel selective alpha 1A receptor agonist. J Med Chem39:4116-9 (1996) [PubMed] Article