| Reaction Details |
|---|
 | Report a problem with these data |
| Target | AP2-associated protein kinase 1 |
|---|
| Ligand | BDBM50273541 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_1806401 (CHEMBL4305760) |
|---|
| Kd | 120±n/a nM |
|---|
| Citation |  Verdonck, S; Pu, SY; Sorrell, FJ; Elkins, JM; Froeyen, M; Gao, LJ; Prugar, LI; Dorosky, DE; Brannan, JM; Barouch-Bentov, R; Knapp, S; Dye, JM; Herdewijn, P; Einav, S; De Jonghe, S Synthesis and Structure-Activity Relationships of 3,5-Disubstituted-pyrrolo[2,3- b]pyridines as Inhibitors of Adaptor-Associated Kinase 1 with Antiviral Activity. J Med Chem62:5810-5831 (2019) [PubMed] Article Verdonck, S; Pu, SY; Sorrell, FJ; Elkins, JM; Froeyen, M; Gao, LJ; Prugar, LI; Dorosky, DE; Brannan, JM; Barouch-Bentov, R; Knapp, S; Dye, JM; Herdewijn, P; Einav, S; De Jonghe, S Synthesis and Structure-Activity Relationships of 3,5-Disubstituted-pyrrolo[2,3- b]pyridines as Inhibitors of Adaptor-Associated Kinase 1 with Antiviral Activity. J Med Chem62:5810-5831 (2019) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| AP2-associated protein kinase 1 |
|---|
| Name: | AP2-associated protein kinase 1 |
|---|
| Synonyms: | AAK1 | AAK1_HUMAN | Adaptor-associated kinase 1 | KIAA1048 |
|---|
| Type: | PROTEIN |
|---|
| Mol. Mass.: | 103884.23 |
|---|
| Organism: | Homo sapiens (Human) |
|---|
| Description: | ChEMBL_774569 |
|---|
| Residue: | 961 |
|---|
| Sequence: | MKKFFDSRREQGGSGLGSGSSGGGGSTSGLGSGYIGRVFGIGRQQVTVDEVLAEGGFAIV
FLVRTSNGMKCALKRMFVNNEHDLQVCKREIQIMRDLSGHKNIVGYIDSSINNVSSGDVW
EVLILMDFCRGGQVVNLMNQRLQTGFTENEVLQIFCDTCEAVARLHQCKTPIIHRDLKVE
NILLHDRGHYVLCDFGSATNKFQNPQTEGVNAVEDEIKKYTTLSYRAPEMVNLYSGKIIT
TKADIWALGCLLYKLCYFTLPFGESQVAICDGNFTIPDNSRYSQDMHCLIRYMLEPDPDK
RPDIYQVSYFSFKLLKKECPIPNVQNSPIPAKLPEPVKASEAAAKKTQPKARLTDPIPTT
ETSIAPRQRPKAGQTQPNPGILPIQPALTPRKRATVQPPPQAAGSSNQPGLLASVPQPKP
QAPPSQPLPQTQAKQPQAPPTPQQTPSTQAQGLPAQAQATPQHQQQLFLKQQQQQQQPPP
AQQQPAGTFYQQQQAQTQQFQAVHPATQKPAIAQFPVVSQGGSQQQLMQNFYQQQQQQQQ
QQQQQQLATALHQQQLMTQQAALQQKPTMAAGQQPQPQPAAAPQPAPAQEPAIQAPVRQQ
PKVQTTPPPAVQGQKVGSLTPPSSPKTQRAGHRRILSDVTHSAVFGVPASKSTQLLQAAA
AEASLNKSKSATTTPSGSPRTSQQNVYNPSEGSTWNPFDDDNFSKLTAEELLNKDFAKLG
EGKHPEKLGGSAESLIPGFQSTQGDAFATTSFSAGTAEKRKGGQTVDSGLPLLSVSDPFI
PLQVPDAPEKLIEGLKSPDTSLLLPDLLPMTDPFGSTSDAVIEKADVAVESLIPGLEPPV
PQRLPSQTESVTSNRTDSLTGEDSLLDCSLLSNPTTDLLEEFAPTAISAPVHKAAEDSNL
ISGFDVPEGSDKVAEDEFDPIPVLITKNPQGGHSRNSSGSSESSLPNLARSLLLVDQLID
L
|
|
|
|---|
| BDBM50273541 |
|---|
| n/a |
|---|
| Name | BDBM50273541 |
|---|
| Synonyms: | CHEMBL516312 | N-(5-(4-cyanophenyl)-1H-pyrrolo[2,3-b]pyridin-3-yl)nicotinamide |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C20H13N5O |
|---|
| Mol. Mass. | 339.3501 |
|---|
| SMILES | O=C(Nc1c[nH]c2ncc(cc12)-c1ccc(cc1)C#N)c1cccnc1 |
|---|
| Structure | 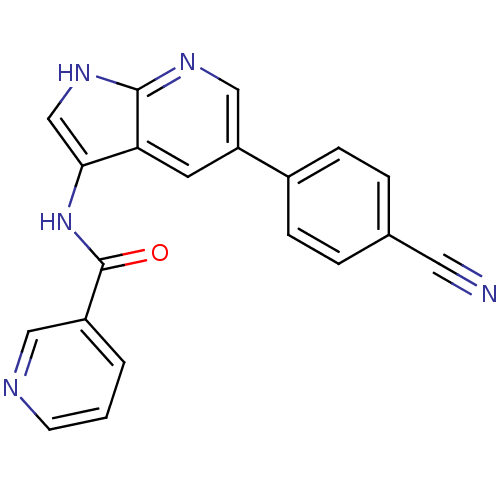
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Verdonck, S; Pu, SY; Sorrell, FJ; Elkins, JM; Froeyen, M; Gao, LJ; Prugar, LI; Dorosky, DE; Brannan, JM; Barouch-Bentov, R; Knapp, S; Dye, JM; Herdewijn, P; Einav, S; De Jonghe, S Synthesis and Structure-Activity Relationships of 3,5-Disubstituted-pyrrolo[2,3- b]pyridines as Inhibitors of Adaptor-Associated Kinase 1 with Antiviral Activity. J Med Chem62:5810-5831 (2019) [PubMed] Article
Verdonck, S; Pu, SY; Sorrell, FJ; Elkins, JM; Froeyen, M; Gao, LJ; Prugar, LI; Dorosky, DE; Brannan, JM; Barouch-Bentov, R; Knapp, S; Dye, JM; Herdewijn, P; Einav, S; De Jonghe, S Synthesis and Structure-Activity Relationships of 3,5-Disubstituted-pyrrolo[2,3- b]pyridines as Inhibitors of Adaptor-Associated Kinase 1 with Antiviral Activity. J Med Chem62:5810-5831 (2019) [PubMed] Article