| Reaction Details |
|---|
 | Report a problem with these data |
| Target | 5-hydroxytryptamine receptor 1A |
|---|
| Ligand | BDBM50036448 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_1200 (CHEMBL615966) |
|---|
| Ki | 271±n/a nM |
|---|
| Citation |  Haadsma-Svensson, SR; Svensson, K; Duncan, N; Smith, MW; Lin, CH C-9 and N-substituted analogs of cis-(3aR)-(-)-2,3,3a,4,5,9b-hexahydro-3- propyl-1H-benz[e]indole-9-carboxamide: 5-HT1A receptor agonists with various degrees of metabolic stability. J Med Chem38:725-34 (1995) [PubMed] Haadsma-Svensson, SR; Svensson, K; Duncan, N; Smith, MW; Lin, CH C-9 and N-substituted analogs of cis-(3aR)-(-)-2,3,3a,4,5,9b-hexahydro-3- propyl-1H-benz[e]indole-9-carboxamide: 5-HT1A receptor agonists with various degrees of metabolic stability. J Med Chem38:725-34 (1995) [PubMed] |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| 5-hydroxytryptamine receptor 1A |
|---|
| Name: | 5-hydroxytryptamine receptor 1A |
|---|
| Synonyms: | 5-HT-1A | 5-HT1 | 5-HT1A | 5-Hydroxytryptamine receptor 1A (5-HT1A) | 5-hydroxytryptamine receptor 1A (5HT1A) | 5HT1A_RAT | 5ht1a | G-21 | Htr1a | Serotonin 1 (5-HT1) receptor | Serotonin 1a (5-HT1a) receptor/Adrenergic receptor alpha-1 | Serotonin receptor 1A |
|---|
| Type: | G Protein-Coupled Receptor (GPCR) |
|---|
| Mol. Mass.: | 46445.29 |
|---|
| Organism: | Rattus norvegicus (rat) |
|---|
| Description: | Binding assays were performed using rat hippocampal membranes. |
|---|
| Residue: | 422 |
|---|
| Sequence: | MDVFSFGQGNNTTASQEPFGTGGNVTSISDVTFSYQVITSLLLGTLIFCAVLGNACVVAA
IALERSLQNVANYLIGSLAVTDLMVSVLVLPMAALYQVLNKWTLGQVTCDLFIALDVLCC
TSSILHLCAIALDRYWAITDPIDYVNKRTPRRAAALISLTWLIGFLISIPPMLGWRTPED
RSDPDACTISKDHGYTIYSTFGAFYIPLLLMLVLYGRIFRAARFRIRKTVRKVEKKGAGT
SLGTSSAPPPKKSLNGQPGSGDWRRCAENRAVGTPCTNGAVRQGDDEATLEVIEVHRVGN
SKEHLPLPSESGSNSYAPACLERKNERNAEAKRKMALARERKTVKTLGIIMGTFILCWLP
FFIVALVLPFCESSCHMPALLGAIINWLGYSNSLLNPVIYAYFNKDFQNAFKKIIKCKFC
RR
|
|
|
|---|
| BDBM50036448 |
|---|
| n/a |
|---|
| Name | BDBM50036448 |
|---|
| Synonyms: | (3aR,9bS)-3-(3-Methyl-but-2-enyl)-2,3,3a,4,5,9b-hexahydro-1H-benzo[e]indole-9-carboxylic acid amide | CHEMBL355601 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C18H24N2O |
|---|
| Mol. Mass. | 284.396 |
|---|
| SMILES | [#6]\[#6](-[#6])=[#6]\[#6]-[#7]-1-[#6]-[#6]-[#6@@H]-2-[#6@H]-1-[#6]-[#6]-c1cccc(-[#6](-[#7])=O)c-21 |
|---|
| Structure | 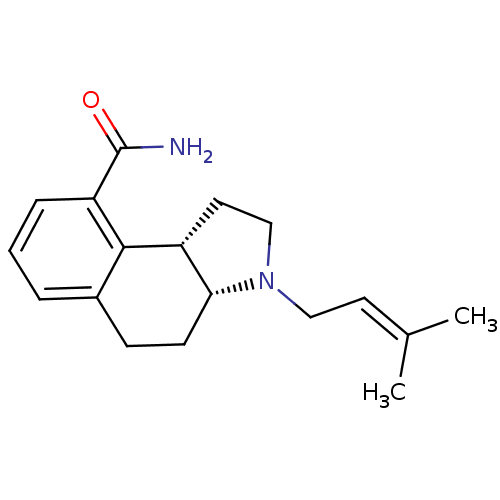
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Haadsma-Svensson, SR; Svensson, K; Duncan, N; Smith, MW; Lin, CH C-9 and N-substituted analogs of cis-(3aR)-(-)-2,3,3a,4,5,9b-hexahydro-3- propyl-1H-benz[e]indole-9-carboxamide: 5-HT1A receptor agonists with various degrees of metabolic stability. J Med Chem38:725-34 (1995) [PubMed]
Haadsma-Svensson, SR; Svensson, K; Duncan, N; Smith, MW; Lin, CH C-9 and N-substituted analogs of cis-(3aR)-(-)-2,3,3a,4,5,9b-hexahydro-3- propyl-1H-benz[e]indole-9-carboxamide: 5-HT1A receptor agonists with various degrees of metabolic stability. J Med Chem38:725-34 (1995) [PubMed]