| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Amine oxidase [flavin-containing] B |
|---|
| Ligand | BDBM50049692 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_124230 (CHEMBL733984) |
|---|
| IC50 | 10000±n/a nM |
|---|
| Citation |  Harfenist, M; Heuser, DJ; Joyner, CT; Batchelor, JF; White, HL Selective inhibitors of monoamine oxidase. 3. Structure-activity relationship of tricyclics bearing imidazoline, oxadiazole, or tetrazole groups. J Med Chem39:1857-63 (1996) [PubMed] Article Harfenist, M; Heuser, DJ; Joyner, CT; Batchelor, JF; White, HL Selective inhibitors of monoamine oxidase. 3. Structure-activity relationship of tricyclics bearing imidazoline, oxadiazole, or tetrazole groups. J Med Chem39:1857-63 (1996) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Amine oxidase [flavin-containing] B |
|---|
| Name: | Amine oxidase [flavin-containing] B |
|---|
| Synonyms: | AOFB_RAT | Amine oxidase (flavin-containing) B | Amine oxidase [flavin-containing] B | Maob | Monoamine Oxidase Type B (MAO-B) | Monoamine oxidase | Monoamine oxidase B (MAO-B) | Monoamine oxidase B (rMAO-B) | Monoamine oxidase type B (MAOB) | Monoamine oxidase-B (MAO-B) |
|---|
| Type: | Enzyme |
|---|
| Mol. Mass.: | 58469.65 |
|---|
| Organism: | Rattus norvegicus (rat) |
|---|
| Description: | P19643 |
|---|
| Residue: | 520 |
|---|
| Sequence: | MSNKCDVIVVGGGISGMAAAKLLHDCGLSVVVLEARDRVGGRTYTIRNKNVKYVDLGGSY
VGPTQNRILRLAKELGLETYKVNEVERLIHFVKGKSYAFRGPFPPVWNPITYLDYNNLWR
TMDEMGQEIPSDAPWKAPLAEEWDYMTMKELLDKICWTNSTKQIATLFVNLCVTAETHEV
SALWFLWYVKQCGGTTRIISTTNGGQERKFIGGSGQVSERIKDILGDRVKLERPVIHIDQ
TGENVVVKTLNHEIYEAKYVISAIPPVLGMKIHHSPPLPILRNQLITRVPLGSVIKCMVY
YKEPFWRKKDFCGTMVIEGEEAPIAYTLDDTKPDGSCAAIMGFILAHKARKLVRLTKEER
LRKLCELYAKVLNSQEALQPVHYEEKNWCEEQYSGGCYTAYFPPGILTQYGRVLRQPVGK
IFFAGTETASHWSGYMEGAVEAGERAAREILHAIGKIPEDEIWQPEPESVDVPARPITNT
FLERHLPSVPGLLKLLGLTTILSATALGFLAHKKGLFVRF
|
|
|
|---|
| BDBM50049692 |
|---|
| n/a |
|---|
| Name | BDBM50049692 |
|---|
| Synonyms: | 2-Phenoxathiin-2-yl-4,5-dihydro-1H-imidazole | CHEMBL301625 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C15H12N2OS |
|---|
| Mol. Mass. | 268.334 |
|---|
| SMILES | C1CN=C(N1)c1ccc2Oc3ccccc3Sc2c1 |c:2| |
|---|
| Structure | 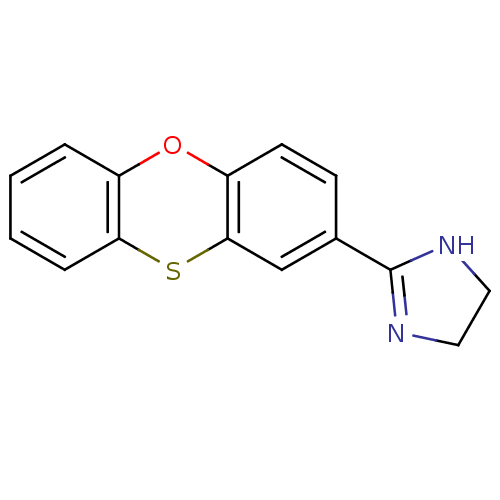
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Harfenist, M; Heuser, DJ; Joyner, CT; Batchelor, JF; White, HL Selective inhibitors of monoamine oxidase. 3. Structure-activity relationship of tricyclics bearing imidazoline, oxadiazole, or tetrazole groups. J Med Chem39:1857-63 (1996) [PubMed] Article
Harfenist, M; Heuser, DJ; Joyner, CT; Batchelor, JF; White, HL Selective inhibitors of monoamine oxidase. 3. Structure-activity relationship of tricyclics bearing imidazoline, oxadiazole, or tetrazole groups. J Med Chem39:1857-63 (1996) [PubMed] Article