| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Acetylcholinesterase |
|---|
| Ligand | BDBM50059905 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_28936 (CHEMBL884109) |
|---|
| IC50 | 100000±n/a nM |
|---|
| Citation |  Boyle, NA; Talesa, V; Giovannini, E; Rosi, G; Norton, SJ Synthesis and study of thiocarbonate derivatives of choline as potential inhibitors of acetylcholinesterase. J Med Chem40:3009-13 (1997) [PubMed] Article Boyle, NA; Talesa, V; Giovannini, E; Rosi, G; Norton, SJ Synthesis and study of thiocarbonate derivatives of choline as potential inhibitors of acetylcholinesterase. J Med Chem40:3009-13 (1997) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Acetylcholinesterase |
|---|
| Name: | Acetylcholinesterase |
|---|
| Synonyms: | ACES_HUMAN | ACHE | Acetylcholinesterase (AChE) | Acetylcholinesterase (human AChE) |
|---|
| Type: | Enzyme |
|---|
| Mol. Mass.: | 67792.70 |
|---|
| Organism: | Homo sapiens (Human) |
|---|
| Description: | P22303 |
|---|
| Residue: | 614 |
|---|
| Sequence: | MRPPQCLLHTPSLASPLLLLLLWLLGGGVGAEGREDAELLVTVRGGRLRGIRLKTPGGPV
SAFLGIPFAEPPMGPRRFLPPEPKQPWSGVVDATTFQSVCYQYVDTLYPGFEGTEMWNPN
RELSEDCLYLNVWTPYPRPTSPTPVLVWIYGGGFYSGASSLDVYDGRFLVQAERTVLVSM
NYRVGAFGFLALPGSREAPGNVGLLDQRLALQWVQENVAAFGGDPTSVTLFGESAGAASV
GMHLLSPPSRGLFHRAVLQSGAPNGPWATVGMGEARRRATQLAHLVGCPPGGTGGNDTEL
VACLRTRPAQVLVNHEWHVLPQESVFRFSFVPVVDGDFLSDTPEALINAGDFHGLQVLVG
VVKDEGSYFLVYGAPGFSKDNESLISRAEFLAGVRVGVPQVSDLAAEAVVLHYTDWLHPE
DPARLREALSDVVGDHNVVCPVAQLAGRLAAQGARVYAYVFEHRASTLSWPLWMGVPHGY
EIEFIFGIPLDPSRNYTAEEKIFAQRLMRYWANFARTGDPNEPRDPKAPQWPPYTAGAQQ
YVSLDLRPLEVRRGLRAQACAFWNRFLPKLLSATDTLDEAERQWKAEFHRWSSYMVHWKN
QFDHYSKQDRCSDL
|
|
|
|---|
| BDBM50059905 |
|---|
| n/a |
|---|
| Name | BDBM50059905 |
|---|
| Synonyms: | (2-Hexylsulfanylcarbonyloxy-ethyl)-trimethyl-ammonium; chloride | CHEMBL102077 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C12H26NO2S |
|---|
| Mol. Mass. | 248.405 |
|---|
| SMILES | CCCCCCSC(=O)OCC[N+](C)(C)C |
|---|
| Structure | 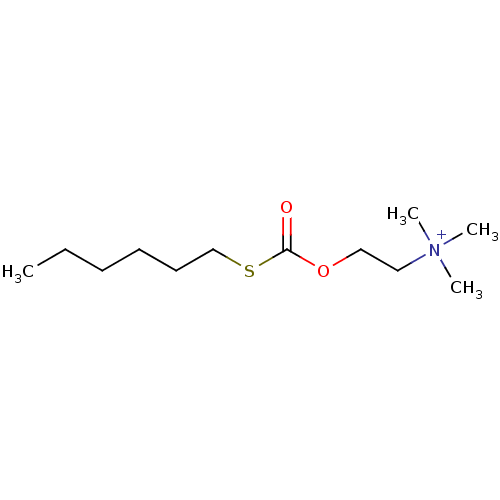
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Boyle, NA; Talesa, V; Giovannini, E; Rosi, G; Norton, SJ Synthesis and study of thiocarbonate derivatives of choline as potential inhibitors of acetylcholinesterase. J Med Chem40:3009-13 (1997) [PubMed] Article
Boyle, NA; Talesa, V; Giovannini, E; Rosi, G; Norton, SJ Synthesis and study of thiocarbonate derivatives of choline as potential inhibitors of acetylcholinesterase. J Med Chem40:3009-13 (1997) [PubMed] Article