| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Beta-glucuronidase |
|---|
| Ligand | BDBM50510749 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_1842064 (CHEMBL4342363) |
|---|
| IC50 | 6200±n/a nM |
|---|
| Citation |  Taha, M; Imran, S; Alomari, M; Rahim, F; Wadood, A; Mosaddik, A; Uddin, N; Gollapalli, M; Alqahtani, MA; Bamarouf, YA Synthesis of oxadiazole-coupled-thiadiazole derivatives as a potent ?-glucuronidase inhibitors and their molecular docking study. Bioorg Med Chem27:3145-3155 (2019) [PubMed] Article Taha, M; Imran, S; Alomari, M; Rahim, F; Wadood, A; Mosaddik, A; Uddin, N; Gollapalli, M; Alqahtani, MA; Bamarouf, YA Synthesis of oxadiazole-coupled-thiadiazole derivatives as a potent ?-glucuronidase inhibitors and their molecular docking study. Bioorg Med Chem27:3145-3155 (2019) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Beta-glucuronidase |
|---|
| Name: | Beta-glucuronidase |
|---|
| Synonyms: | β-Glucuronidase | BGLR_HUMAN | Beta-G1 | Beta-glucuronidase | GUSB | beta-Glucuronidase (β-glucuronidase) |
|---|
| Type: | Protein |
|---|
| Mol. Mass.: | 74736.05 |
|---|
| Organism: | Homo sapiens (Human) |
|---|
| Description: | n/a |
|---|
| Residue: | 651 |
|---|
| Sequence: | MARGSAVAWAALGPLLWGCALGLQGGMLYPQESPSRECKELDGLWSFRADFSDNRRRGFE
EQWYRRPLWESGPTVDMPVPSSFNDISQDWRLRHFVGWVWYEREVILPERWTQDLRTRVV
LRIGSAHSYAIVWVNGVDTLEHEGGYLPFEADISNLVQVGPLPSRLRITIAINNTLTPTT
LPPGTIQYLTDTSKYPKGYFVQNTYFDFFNYAGLQRSVLLYTTPTTYIDDITVTTSVEQD
SGLVNYQISVKGSNLFKLEVRLLDAENKVVANGTGTQGQLKVPGVSLWWPYLMHERPAYL
YSLEVQLTAQTSLGPVSDFYTLPVGIRTVAVTKSQFLINGKPFYFHGVNKHEDADIRGKG
FDWPLLVKDFNLLRWLGANAFRTSHYPYAEEVMQMCDRYGIVVIDECPGVGLALPQFFNN
VSLHHHMQVMEEVVRRDKNHPAVVMWSVANEPASHLESAGYYLKMVIAHTKSLDPSRPVT
FVSNSNYAADKGAPYVDVICLNSYYSWYHDYGHLELIQLQLATQFENWYKKYQKPIIQSE
YGAETIAGFHQDPPLMFTEEYQKSLLEQYHLGLDQKRRKYVVGELIWNFADFMTEQSPTR
VLGNKKGIFTRQRQPKSAAFLLRERYWKIANETRYPHSVAKSQCLENSLFT
|
|
|
|---|
| BDBM50510749 |
|---|
| n/a |
|---|
| Name | BDBM50510749 |
|---|
| Synonyms: | CHEMBL4565724 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C23H16N4O5S |
|---|
| Mol. Mass. | 460.462 |
|---|
| SMILES | COc1cc(O)ccc1-c1nnc(o1)-c1ccc(cc1)-c1nnc(s1)-c1cc(O)ccc1O |
|---|
| Structure | 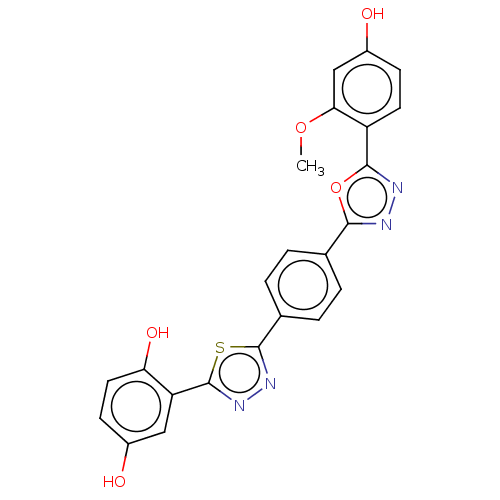
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Taha, M; Imran, S; Alomari, M; Rahim, F; Wadood, A; Mosaddik, A; Uddin, N; Gollapalli, M; Alqahtani, MA; Bamarouf, YA Synthesis of oxadiazole-coupled-thiadiazole derivatives as a potent ?-glucuronidase inhibitors and their molecular docking study. Bioorg Med Chem27:3145-3155 (2019) [PubMed] Article
Taha, M; Imran, S; Alomari, M; Rahim, F; Wadood, A; Mosaddik, A; Uddin, N; Gollapalli, M; Alqahtani, MA; Bamarouf, YA Synthesis of oxadiazole-coupled-thiadiazole derivatives as a potent ?-glucuronidase inhibitors and their molecular docking study. Bioorg Med Chem27:3145-3155 (2019) [PubMed] Article