| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Tyrosine-protein kinase JAK3 |
|---|
| Ligand | BDBM214689 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_1842479 (CHEMBL4342906) |
|---|
| Kd | 5.0±n/a nM |
|---|
| Citation |  Das, D; Hong, J Recent advancements of 4-aminoquinazoline derivatives as kinase inhibitors and their applications in medicinal chemistry. Eur J Med Chem170:55-72 (2019) [PubMed] Article Das, D; Hong, J Recent advancements of 4-aminoquinazoline derivatives as kinase inhibitors and their applications in medicinal chemistry. Eur J Med Chem170:55-72 (2019) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Tyrosine-protein kinase JAK3 |
|---|
| Name: | Tyrosine-protein kinase JAK3 |
|---|
| Synonyms: | JAK-3 | JAK3 | JAK3_HUMAN | Janus kinase 3 | Janus kinase 3 (JAK3) | Janus kinase 3 JAK3 | L-JAK | Leukocyte janus kinase |
|---|
| Type: | Protein |
|---|
| Mol. Mass.: | 125111.08 |
|---|
| Organism: | Homo sapiens (Human) |
|---|
| Description: | P52333 |
|---|
| Residue: | 1124 |
|---|
| Sequence: | MAPPSEETPLIPQRSCSLLSTEAGALHVLLPARGPGPPQRLSFSFGDHLAEDLCVQAAKA
SGILPVYHSLFALATEDLSCWFPPSHIFSVEDASTQVLLYRIRFYFPNWFGLEKCHRFGL
RKDLASAILDLPVLEHLFAQHRSDLVSGRLPVGLSLKEQGECLSLAVLDLARMAREQAQR
PGELLKTVSYKACLPPSLRDLIQGLSFVTRRRIRRTVRRALRRVAACQADRHSLMAKYIM
DLERLDPAGAAETFHVGLPGALGGHDGLGLLRVAGDGGIAWTQGEQEVLQPFCDFPEIVD
ISIKQAPRVGPAGEHRLVTVTRTDNQILEAEFPGLPEALSFVALVDGYFRLTTDSQHFFC
KEVAPPRLLEEVAEQCHGPITLDFAINKLKTGGSRPGSYVLRRSPQDFDSFLLTVCVQNP
LGPDYKGCLIRRSPTGTFLLVGLSRPHSSLRELLATCWDGGLHVDGVAVTLTSCCIPRPK
EKSNLIVVQRGHSPPTSSLVQPQSQYQLSQMTFHKIPADSLEWHENLGHGSFTKIYRGCR
HEVVDGEARKTEVLLKVMDAKHKNCMESFLEAASLMSQVSYRHLVLLHGVCMAGDSTMVQ
EFVHLGAIDMYLRKRGHLVPASWKLQVVKQLAYALNYLEDKGLPHGNVSARKVLLAREGA
DGSPPFIKLSDPGVSPAVLSLEMLTDRIPWVAPECLREAQTLSLEADKWGFGATVWEVFS
GVTMPISALDPAKKLQFYEDRQQLPAPKWTELALLIQQCMAYEPVQRPSFRAVIRDLNSL
ISSDYELLSDPTPGALAPRDGLWNGAQLYACQDPTIFEERHLKYISQLGKGNFGSVELCR
YDPLGDNTGALVAVKQLQHSGPDQQRDFQREIQILKALHSDFIVKYRGVSYGPGRQSLRL
VMEYLPSGCLRDFLQRHRARLDASRLLLYSSQICKGMEYLGSRRCVHRDLAARNILVESE
AHVKIADFGLAKLLPLDKDYYVVREPGQSPIFWYAPESLSDNIFSRQSDVWSFGVVLYEL
FTYCDKSCSPSAEFLRMMGCERDVPALCRLLELLEEGQRLPAPPACPAEVHELMKLCWAP
SPQDRPSFSALGPQLDMLWSGSRGCETHAFTAHPEGKHHSLSFS
|
|
|
|---|
| BDBM214689 |
|---|
| n/a |
|---|
| Name | BDBM214689 |
|---|
| Synonyms: | US9295672, (S)-(4-fluorophenyl)(4-((5-methyl-1H-pyrazol-3-yl)amino)quinazolin-2-yl)methanol |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C19H16FN5O |
|---|
| Mol. Mass. | 349.3616 |
|---|
| SMILES | Cc1cc(Nc2nc(nc3ccccc23)[C@@H](O)c2ccc(F)cc2)n[nH]1 |
|---|
| Structure | 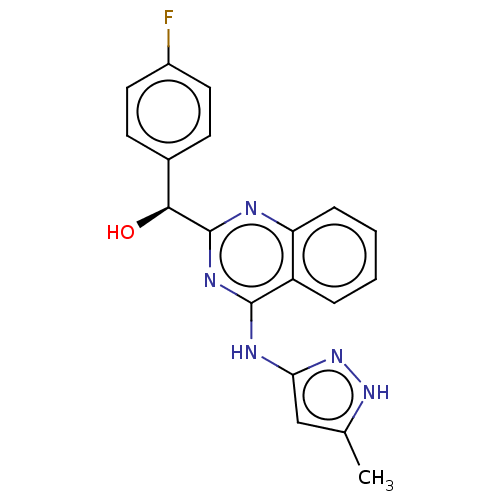
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Das, D; Hong, J Recent advancements of 4-aminoquinazoline derivatives as kinase inhibitors and their applications in medicinal chemistry. Eur J Med Chem170:55-72 (2019) [PubMed] Article
Das, D; Hong, J Recent advancements of 4-aminoquinazoline derivatives as kinase inhibitors and their applications in medicinal chemistry. Eur J Med Chem170:55-72 (2019) [PubMed] Article