| Reaction Details |
|---|
 | Report a problem with these data |
| Target | GTPase KRas |
|---|
| Ligand | BDBM50519444 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_1875329 (CHEMBL4376618) |
|---|
| Kd | 870±n/a nM |
|---|
| Citation |  Xu, LL; Li, CC; An, LY; Dai, Z; Chen, XY; You, QD; Hu, C; Di, B Selective apoptosis-inducing activity of synthetic hydrocarbon-stapled SOS1 helix with d-amino acids in H358 cancer cells expressing KRAS Eur J Med Chem185:0 (2020) [PubMed] Article Xu, LL; Li, CC; An, LY; Dai, Z; Chen, XY; You, QD; Hu, C; Di, B Selective apoptosis-inducing activity of synthetic hydrocarbon-stapled SOS1 helix with d-amino acids in H358 cancer cells expressing KRAS Eur J Med Chem185:0 (2020) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| GTPase KRas |
|---|
| Name: | GTPase KRas |
|---|
| Synonyms: | GTPase KRas, N-terminally processed | K-Ras 2 | KRAS | KRAS2 | Ki-Ras | RASK2 | RASK_HUMAN | c-K-ras | c-Ki-ras |
|---|
| Type: | PROTEIN |
|---|
| Mol. Mass.: | 21656.10 |
|---|
| Organism: | Homo sapiens (Human) |
|---|
| Description: | ChEMBL_1476955 |
|---|
| Residue: | 189 |
|---|
| Sequence: | MTEYKLVVVGAGGVGKSALTIQLIQNHFVDEYDPTIEDSYRKQVVIDGETCLLDILDTAG
QEEYSAMRDQYMRTGEGFLCVFAINNTKSFEDIHHYREQIKRVKDSEDVPMVLVGNKCDL
PSRTVDTKQAQDLARSYGIPFIETSAKTRQRVEDAFYTLVREIRQYRLKKISKEEKTPGC
VKIKKCIIM
|
|
|
|---|
| BDBM50519444 |
|---|
| n/a |
|---|
| Name | BDBM50519444 |
|---|
| Synonyms: | CHEMBL4567024 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C108H169N29O34 |
|---|
| Mol. Mass. | 2417.6714 |
|---|
| SMILES | CC[C@H](C)[C@H](NC(=O)CNC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CCCNC(N)=N)NC(C)=O)C(=O)N[C@@]1(C)CCC\C=C/CCC[C@](C)(NC(=O)[C@H](CC(N)=O)NC(=O)[C@H](CC(O)=O)NC(=O)[C@@H](NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CC(O)=O)NC1=O)[C@@H](C)O)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CCC(O)=O)C(=O)NCC(=O)N[C@@H](CC(N)=O)C(O)=O |r,c:67| |
|---|
| Structure | 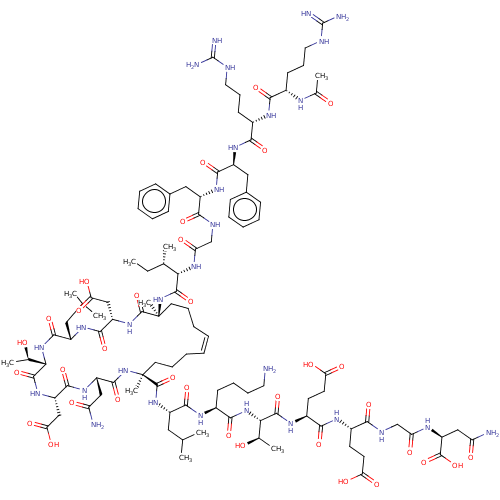
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Xu, LL; Li, CC; An, LY; Dai, Z; Chen, XY; You, QD; Hu, C; Di, B Selective apoptosis-inducing activity of synthetic hydrocarbon-stapled SOS1 helix with d-amino acids in H358 cancer cells expressing KRAS Eur J Med Chem185:0 (2020) [PubMed] Article
Xu, LL; Li, CC; An, LY; Dai, Z; Chen, XY; You, QD; Hu, C; Di, B Selective apoptosis-inducing activity of synthetic hydrocarbon-stapled SOS1 helix with d-amino acids in H358 cancer cells expressing KRAS Eur J Med Chem185:0 (2020) [PubMed] Article