| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Adenosine receptor A3 |
|---|
| Ligand | BDBM50053929 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_31989 (CHEMBL646437) |
|---|
| Ki | 0.65±n/a nM |
|---|
| Citation |  Baraldi, PG; Cacciari, B; Romagnoli, R; Spalluto, G; Klotz, KN; Leung, E; Varani, K; Gessi, S; Merighi, S; Borea, PA Pyrazolo[4,3-e]-1,2,4-triazolo[1,5-c]pyrimidine derivatives as highly potent and selective human A(3) adenosine receptor antagonists. J Med Chem42:4473-8 (1999) [PubMed] Baraldi, PG; Cacciari, B; Romagnoli, R; Spalluto, G; Klotz, KN; Leung, E; Varani, K; Gessi, S; Merighi, S; Borea, PA Pyrazolo[4,3-e]-1,2,4-triazolo[1,5-c]pyrimidine derivatives as highly potent and selective human A(3) adenosine receptor antagonists. J Med Chem42:4473-8 (1999) [PubMed] |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Adenosine receptor A3 |
|---|
| Name: | Adenosine receptor A3 |
|---|
| Synonyms: | A3 adenosine receptor (hA3) | AA3R_HUMAN | ADORA3 | Adenosine A3 receptor (A3AR) |
|---|
| Type: | G Protein-Coupled Receptor (GPCR) |
|---|
| Mol. Mass.: | 36197.32 |
|---|
| Organism: | Homo sapiens (Human) |
|---|
| Description: | P0DMS8 |
|---|
| Residue: | 318 |
|---|
| Sequence: | MPNNSTALSLANVTYITMEIFIGLCAIVGNVLVICVVKLNPSLQTTTFYFIVSLALADIA
VGVLVMPLAIVVSLGITIHFYSCLFMTCLLLIFTHASIMSLLAIAVDRYLRVKLTVRYKR
VTTHRRIWLALGLCWLVSFLVGLTPMFGWNMKLTSEYHRNVTFLSCQFVSVMRMDYMVYF
SFLTWIFIPLVVMCAIYLDIFYIIRNKLSLNLSNSKETGAFYGREFKTAKSLFLVLFLFA
LSWLPLSIINCIIYFNGEVPQLVLYMGILLSHANSMMNPIVYAYKIKKFKETYLLILKAC
VVCHPSDSLDTSIEKNSE
|
|
|
|---|
| BDBM50053929 |
|---|
| n/a |
|---|
| Name | BDBM50053929 |
|---|
| Synonyms: | CHEMBL88147 | N-(9-Chloro-2-furan-2-yl-[1,2,4]triazolo[1,5-c]quinazolin-5-yl)-2-phenyl-acetamide | N-(9-Chloro-2-furan-2-yl-[1,2,4]triazolo[1,5-c]quinazolin-5-yl)-N-methyl-2-phenyl-acetamide | N-(9-chloro-2-(furan-2-yl)-[1,2,4]triazolo[1,5-c]quinazolin-5-yl)-2-phenylacetamide |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C21H14ClN5O2 |
|---|
| Mol. Mass. | 403.821 |
|---|
| SMILES | Clc1ccc2nc(NC(=O)Cc3ccccc3)n3nc(nc3c2c1)-c1ccco1 |
|---|
| Structure | 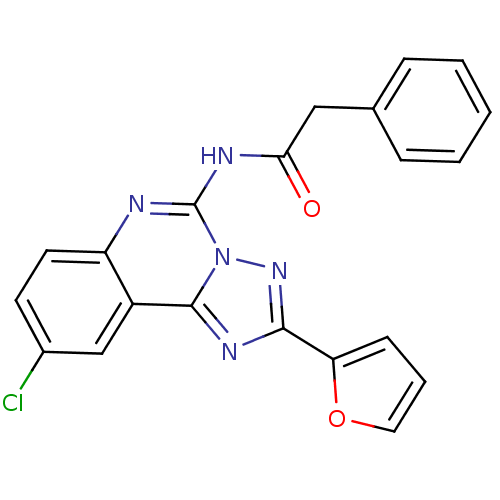
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Baraldi, PG; Cacciari, B; Romagnoli, R; Spalluto, G; Klotz, KN; Leung, E; Varani, K; Gessi, S; Merighi, S; Borea, PA Pyrazolo[4,3-e]-1,2,4-triazolo[1,5-c]pyrimidine derivatives as highly potent and selective human A(3) adenosine receptor antagonists. J Med Chem42:4473-8 (1999) [PubMed]
Baraldi, PG; Cacciari, B; Romagnoli, R; Spalluto, G; Klotz, KN; Leung, E; Varani, K; Gessi, S; Merighi, S; Borea, PA Pyrazolo[4,3-e]-1,2,4-triazolo[1,5-c]pyrimidine derivatives as highly potent and selective human A(3) adenosine receptor antagonists. J Med Chem42:4473-8 (1999) [PubMed]