| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Alpha-1A adrenergic receptor |
|---|
| Ligand | BDBM50090027 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_33604 (CHEMBL652812) |
|---|
| Ki | 0.18±n/a nM |
|---|
| Citation |  Barrow, JC; Nantermet, PG; Selnick, HG; Glass, KL; Rittle, KE; Gilbert, KF; Steele, TG; Homnick, CF; Freidinger, RM; Ransom, RW; Kling, P; Reiss, D; Broten, TP; Schorn, TW; Chang, RS; O'Malley, SS; Olah, TV; Ellis, JD; Barrish, A; Kassahun, K; Leppert, P; Nagarathnam, D; Forray, C In vitro and in vivo evaluation of dihydropyrimidinone C-5 amides as potent and selective alpha(1A) receptor antagonists for the treatment of benign prostatic hyperplasia. J Med Chem43:2703-18 (2000) [PubMed] Barrow, JC; Nantermet, PG; Selnick, HG; Glass, KL; Rittle, KE; Gilbert, KF; Steele, TG; Homnick, CF; Freidinger, RM; Ransom, RW; Kling, P; Reiss, D; Broten, TP; Schorn, TW; Chang, RS; O'Malley, SS; Olah, TV; Ellis, JD; Barrish, A; Kassahun, K; Leppert, P; Nagarathnam, D; Forray, C In vitro and in vivo evaluation of dihydropyrimidinone C-5 amides as potent and selective alpha(1A) receptor antagonists for the treatment of benign prostatic hyperplasia. J Med Chem43:2703-18 (2000) [PubMed] |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Alpha-1A adrenergic receptor |
|---|
| Name: | Alpha-1A adrenergic receptor |
|---|
| Synonyms: | ADA1A_HUMAN | ADRA1A | ADRA1C | Adrenergic alpha1A | Alpha 1A-adrenoceptor | Alpha 1A-adrenoreceptor | Alpha adrenergic receptor 1a | Alpha-1C adrenergic receptor | Alpha-adrenergic receptor 1c | Cerebral cortex alpha adrenergic receptor | adrenergic Alpha1 | adrenergic Alpha1C |
|---|
| Type: | Cell-surface receptors |
|---|
| Mol. Mass.: | 51511.67 |
|---|
| Organism: | Homo sapiens (Human) |
|---|
| Description: | P35348 |
|---|
| Residue: | 466 |
|---|
| Sequence: | MVFLSGNASDSSNCTQPPAPVNISKAILLGVILGGLILFGVLGNILVILSVACHRHLHSV
THYYIVNLAVADLLLTSTVLPFSAIFEVLGYWAFGRVFCNIWAAVDVLCCTASIMGLCII
SIDRYIGVSYPLRYPTIVTQRRGLMALLCVWALSLVISIGPLFGWRQPAPEDETICQINE
EPGYVLFSALGSFYLPLAIILVMYCRVYVVAKRESRGLKSGLKTDKSDSEQVTLRIHRKN
APAGGSGMASAKTKTHFSVRLLKFSREKKAAKTLGIVVGCFVLCWLPFFLVMPIGSFFPD
FKPSETVFKIVFWLGYLNSCINPIIYPCSSQEFKKAFQNVLRIQCLCRKQSSKHALGYTL
HPPSQAVEGQHKDMVRIPVGSRETFYRISKTDGVCEWKFFSSMPRGSARITVSKDQSSCT
TARVRSKSFLQVCCCVGPSTPSLDKNHQVPTIKVHTISLSENGEEV
|
|
|
|---|
| BDBM50090027 |
|---|
| n/a |
|---|
| Name | BDBM50090027 |
|---|
| Synonyms: | (R)-4-(3,4-Difluoro-phenyl)-1,3,6-trimethyl-2-oxo-1,2,3,4-tetrahydro-pyrimidine-5-carboxylic acid {3-[4-(4-fluoro-phenyl)-piperidin-1-yl]-propyl}-amide | CHEMBL90420 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C28H33F3N4O2 |
|---|
| Mol. Mass. | 514.5824 |
|---|
| SMILES | CN1[C@@H](C(C(=O)NCCCN2CCC(CC2)c2ccc(F)cc2)=C(C)N(C)C1=O)c1ccc(F)c(F)c1 |t:24| |
|---|
| Structure | 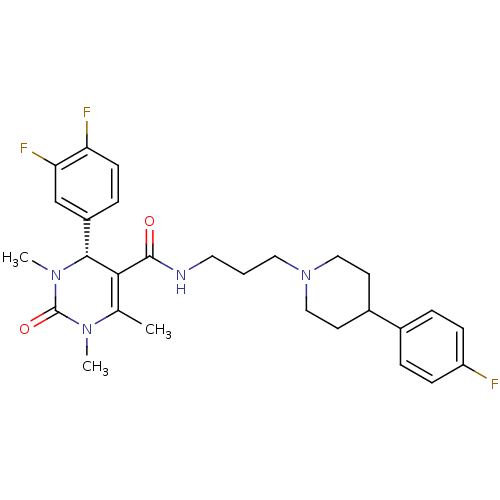
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Barrow, JC; Nantermet, PG; Selnick, HG; Glass, KL; Rittle, KE; Gilbert, KF; Steele, TG; Homnick, CF; Freidinger, RM; Ransom, RW; Kling, P; Reiss, D; Broten, TP; Schorn, TW; Chang, RS; O'Malley, SS; Olah, TV; Ellis, JD; Barrish, A; Kassahun, K; Leppert, P; Nagarathnam, D; Forray, C In vitro and in vivo evaluation of dihydropyrimidinone C-5 amides as potent and selective alpha(1A) receptor antagonists for the treatment of benign prostatic hyperplasia. J Med Chem43:2703-18 (2000) [PubMed]
Barrow, JC; Nantermet, PG; Selnick, HG; Glass, KL; Rittle, KE; Gilbert, KF; Steele, TG; Homnick, CF; Freidinger, RM; Ransom, RW; Kling, P; Reiss, D; Broten, TP; Schorn, TW; Chang, RS; O'Malley, SS; Olah, TV; Ellis, JD; Barrish, A; Kassahun, K; Leppert, P; Nagarathnam, D; Forray, C In vitro and in vivo evaluation of dihydropyrimidinone C-5 amides as potent and selective alpha(1A) receptor antagonists for the treatment of benign prostatic hyperplasia. J Med Chem43:2703-18 (2000) [PubMed]