| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Leukotriene A-4 hydrolase |
|---|
| Ligand | BDBM50541953 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_1985687 (CHEMBL4619093) |
|---|
| IC50 | 750±n/a nM |
|---|
| Citation |  Hefke, L; Hiesinger, K; Zhu, WF; Kramer, JS; Proschak, E Computer-Aided Fragment Growing Strategies to Design Dual Inhibitors of Soluble Epoxide Hydrolase and LTA4 Hydrolase. ACS Med Chem Lett11:1244-1249 (2020) [PubMed] Article Hefke, L; Hiesinger, K; Zhu, WF; Kramer, JS; Proschak, E Computer-Aided Fragment Growing Strategies to Design Dual Inhibitors of Soluble Epoxide Hydrolase and LTA4 Hydrolase. ACS Med Chem Lett11:1244-1249 (2020) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Leukotriene A-4 hydrolase |
|---|
| Name: | Leukotriene A-4 hydrolase |
|---|
| Synonyms: | LKHA4_HUMAN | LTA-4 hydrolase | LTA4 | LTA4H | Leukotriene A(4) hydrolase | Leukotriene A-4 hydrolase (LTA4H) | Leukotriene A4 hydrolase |
|---|
| Type: | Hydrolase; metalloprotease |
|---|
| Mol. Mass.: | 69280.41 |
|---|
| Organism: | Homo sapiens (Human) |
|---|
| Description: | Human recombinant LTA4H. |
|---|
| Residue: | 611 |
|---|
| Sequence: | MPEIVDTCSLASPASVCRTKHLHLRCSVDFTRRTLTGTAALTVQSQEDNLRSLVLDTKDL
TIEKVVINGQEVKYALGERQSYKGSPMEISLPIALSKNQEIVIEISFETSPKSSALQWLT
PEQTSGKEHPYLFSQCQAIHCRAILPCQDTPSVKLTYTAEVSVPKELVALMSAIRDGETP
DPEDPSRKIYKFIQKVPIPCYLIALVVGALESRQIGPRTLVWSEKEQVEKSAYEFSETES
MLKIAEDLGGPYVWGQYDLLVLPPSFPYGGMENPCLTFVTPTLLAGDKSLSNVIAHEISH
SWTGNLVTNKTWDHFWLNEGHTVYLERHICGRLFGEKFRHFNALGGWGELQNSVKTFGET
HPFTKLVVDLTDIDPDVAYSSVPYEKGFALLFYLEQLLGGPEIFLGFLKAYVEKFSYKSI
TTDDWKDFLYSYFKDKVDVLNQVDWNAWLYSPGLPPIKPNYDMTLTNACIALSQRWITAK
EDDLNSFNATDLKDLSSHQLNEFLAQTLQRAPLPLGHIKRMQEVYNFNAINNSEIRFRWL
RLCIQSKWEDAIPLALKMATEQGRMKFTRPLFKDLAAFDKSHDQAVRTYQEHKASMHPVT
AMLVGKDLKVD
|
|
|
|---|
| BDBM50541953 |
|---|
| n/a |
|---|
| Name | BDBM50541953 |
|---|
| Synonyms: | CHEMBL4644372 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C27H32N2O2S |
|---|
| Mol. Mass. | 448.62 |
|---|
| SMILES | O=C(CCc1ccc(OCc2ccccc2)cc1)NCC1CCN(Cc2cccs2)CC1 |
|---|
| Structure | 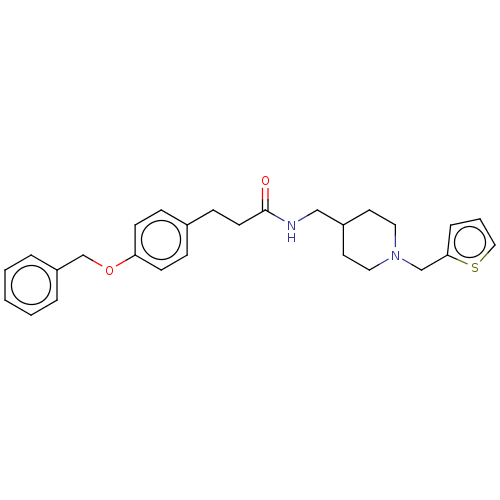
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Hefke, L; Hiesinger, K; Zhu, WF; Kramer, JS; Proschak, E Computer-Aided Fragment Growing Strategies to Design Dual Inhibitors of Soluble Epoxide Hydrolase and LTA4 Hydrolase. ACS Med Chem Lett11:1244-1249 (2020) [PubMed] Article
Hefke, L; Hiesinger, K; Zhu, WF; Kramer, JS; Proschak, E Computer-Aided Fragment Growing Strategies to Design Dual Inhibitors of Soluble Epoxide Hydrolase and LTA4 Hydrolase. ACS Med Chem Lett11:1244-1249 (2020) [PubMed] Article