| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Sterol O-acyltransferase 1 |
|---|
| Ligand | BDBM50241945 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_1994031 (CHEMBL4627926) |
|---|
| IC50 | 174900±n/a nM |
|---|
| Citation |  Nur, EAA; Ohshiro, T; Kobayashi, K; Wu, J; Wahyudin, E; Zhang, H; Hayashi, F; Kawagishi, H; Tomoda, H Inhibition of cholesteryl ester synthesis by polyacetylenes from Atractylodes rhizome. Bioorg Med Chem Lett30:0 (2020) [PubMed] Article Nur, EAA; Ohshiro, T; Kobayashi, K; Wu, J; Wahyudin, E; Zhang, H; Hayashi, F; Kawagishi, H; Tomoda, H Inhibition of cholesteryl ester synthesis by polyacetylenes from Atractylodes rhizome. Bioorg Med Chem Lett30:0 (2020) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Sterol O-acyltransferase 1 |
|---|
| Name: | Sterol O-acyltransferase 1 |
|---|
| Synonyms: | ACACT | ACACT1 | ACAT | ACAT1 | Acetyl-CoA acetyltransferase, mitochondrial | Acyl coenzyme A:cholesterol acyltransferase 1 | Acyl-CoA: cholesterol acyltransferase (ACAT) | Acyl-coenzyme A:cholesterol acyltransferase 1 (ACAT1) | Cholesterol acyltransferase 1 | SOAT | SOAT1 | SOAT1_HUMAN | STAT |
|---|
| Type: | Multi-pass membrane protein may form homo- or heterodimers. |
|---|
| Mol. Mass.: | 64751.94 |
|---|
| Organism: | Homo sapiens (Human) |
|---|
| Description: | P35610 |
|---|
| Residue: | 550 |
|---|
| Sequence: | MVGEEKMSLRNRLSKSRENPEEDEDQRNPAKESLETPSNGRIDIKQLIAKKIKLTAEAEE
LKPFFMKEVGSHFDDFVTNLIEKSASLDNGGCALTTFSVLEGEKNNHRAKDLRAPPEQGK
IFIARRSLLDELLEVDHIRTIYHMFIALLILFILSTLVVDYIDEGRLVLEFSLLSYAFGK
FPTVVWTWWIMFLSTFSVPYFLFQHWATGYSKSSHPLIRSLFHGFLFMIFQIGVLGFGPT
YVVLAYTLPPASRFIIIFEQIRFVMKAHSFVRENVPRVLNSAKEKSSTVPIPTVNQYLYF
LFAPTLIYRDSYPRNPTVRWGYVAMKFAQVFGCFFYVYYIFERLCAPLFRNIKQEPFSAR
VLVLCVFNSILPGVLILFLTFFAFLHCWLNAFAEMLRFGDRMFYKDWWNSTSYSNYYRTW
NVVVHDWLYYYAYKDFLWFFSKRFKSAAMLAVFAVSAVVHEYALAVCLSFFYPVLFVLFM
FFGMAFNFIVNDSRKKPIWNVLMWTSLFLGNGVLLCFYSQEWYARQHCPLKNPTFLDYVR
PRSWTCRYVF
|
|
|
|---|
| BDBM50241945 |
|---|
| n/a |
|---|
| Name | BDBM50241945 |
|---|
| Synonyms: | Atractylenolide III | CHEMBL486961 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C15H20O3 |
|---|
| Mol. Mass. | 248.3175 |
|---|
| SMILES | CC1=C2C[C@H]3C(=C)CCC[C@]3(C)C[C@]2(O)OC1=O |r,c:1| |
|---|
| Structure | 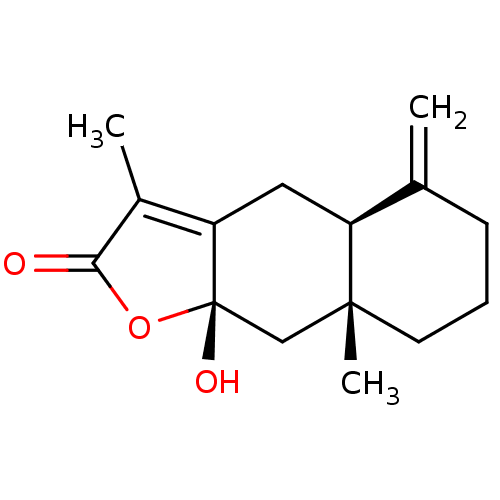
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Nur, EAA; Ohshiro, T; Kobayashi, K; Wu, J; Wahyudin, E; Zhang, H; Hayashi, F; Kawagishi, H; Tomoda, H Inhibition of cholesteryl ester synthesis by polyacetylenes from Atractylodes rhizome. Bioorg Med Chem Lett30:0 (2020) [PubMed] Article
Nur, EAA; Ohshiro, T; Kobayashi, K; Wu, J; Wahyudin, E; Zhang, H; Hayashi, F; Kawagishi, H; Tomoda, H Inhibition of cholesteryl ester synthesis by polyacetylenes from Atractylodes rhizome. Bioorg Med Chem Lett30:0 (2020) [PubMed] Article