| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Procathepsin L |
|---|
| Ligand | BDBM50108862 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_48504 (CHEMBL660653) |
|---|
| Ki | 1.7±n/a nM |
|---|
| Citation |  Huang, L; Lee, A; Ellman, JA Identification of potent and selective mechanism-based inhibitors of the cysteine protease cruzain using solid-phase parallel synthesis. J Med Chem45:676-84 (2002) [PubMed] Huang, L; Lee, A; Ellman, JA Identification of potent and selective mechanism-based inhibitors of the cysteine protease cruzain using solid-phase parallel synthesis. J Med Chem45:676-84 (2002) [PubMed] |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Procathepsin L |
|---|
| Name: | Procathepsin L |
|---|
| Synonyms: | CATL1_HUMAN | CTSL | CTSL CTSL1 | CTSL1 | Cathepsin L | Cathepsin L1 | Cathepsin L1 heavy chain | Cathepsin L1 light chain | MEP | Major excreted protein | cathepsin L preproprotein |
|---|
| Type: | Enzyme |
|---|
| Mol. Mass.: | 37557.19 |
|---|
| Organism: | Homo sapiens (Human) |
|---|
| Description: | Purchased from Calbiochem (San Diego, CA). |
|---|
| Residue: | 333 |
|---|
| Sequence: | MNPTLILAAFCLGIASATLTFDHSLEAQWTKWKAMHNRLYGMNEEGWRRAVWEKNMKMIE
LHNQEYREGKHSFTMAMNAFGDMTSEEFRQVMNGFQNRKPRKGKVFQEPLFYEAPRSVDW
REKGYVTPVKNQGQCGSCWAFSATGALEGQMFRKTGRLISLSEQNLVDCSGPQGNEGCNG
GLMDYAFQYVQDNGGLDSEESYPYEATEESCKYNPKYSVANDTGFVDIPKQEKALMKAVA
TVGPISVAIDAGHESFLFYKEGIYFEPDCSSEDMDHGVLVVGYGFESTESDNNKYWLVKN
SWGEEWGMGGYVKMAKDRRNHCGIASAASYPTV
|
|
|
|---|
| BDBM50108862 |
|---|
| n/a |
|---|
| Name | BDBM50108862 |
|---|
| Synonyms: | CHEMBL161651 | [1-(1-Benzyl-3-tert-butylsulfanyl-2-oxo-propylcarbamoyl)-2-phenyl-ethyl]-carbamic acid benzyl ester | benzyl (S)-1-((S)-4-(tert-butylthio)-3-oxo-1-phenylbutan-2-ylamino)-1-oxo-3-phenylpropan-2-ylcarbamate |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C31H36N2O4S |
|---|
| Mol. Mass. | 532.694 |
|---|
| SMILES | CC(C)(C)SCC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](Cc1ccccc1)NC(=O)OCc1ccccc1 |
|---|
| Structure | 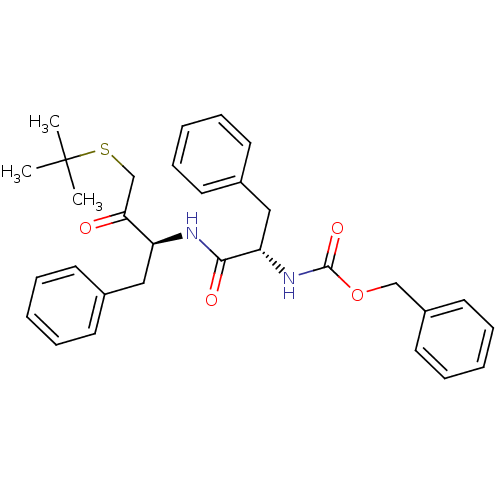
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Huang, L; Lee, A; Ellman, JA Identification of potent and selective mechanism-based inhibitors of the cysteine protease cruzain using solid-phase parallel synthesis. J Med Chem45:676-84 (2002) [PubMed]
Huang, L; Lee, A; Ellman, JA Identification of potent and selective mechanism-based inhibitors of the cysteine protease cruzain using solid-phase parallel synthesis. J Med Chem45:676-84 (2002) [PubMed]