| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Histone deacetylase 1 |
|---|
| Ligand | BDBM50100456 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_87865 (CHEMBL697288) |
|---|
| IC50 | 4500±n/a nM |
|---|
| Citation |  Mai, A; Massa, S; Ragno, R; Esposito, M; Sbardella, G; Nocca, G; Scatena, R; Jesacher, F; Loidl, P; Brosch, G Binding mode analysis of 3-(4-benzoyl-1-methyl-1H-2-pyrrolyl)-N-hydroxy-2-propenamide: a new synthetic histone deacetylase inhibitor inducing histone hyperacetylation, growth inhibition, and terminal cell differentiation. J Med Chem45:1778-84 (2002) [PubMed] Mai, A; Massa, S; Ragno, R; Esposito, M; Sbardella, G; Nocca, G; Scatena, R; Jesacher, F; Loidl, P; Brosch, G Binding mode analysis of 3-(4-benzoyl-1-methyl-1H-2-pyrrolyl)-N-hydroxy-2-propenamide: a new synthetic histone deacetylase inhibitor inducing histone hyperacetylation, growth inhibition, and terminal cell differentiation. J Med Chem45:1778-84 (2002) [PubMed] |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Histone deacetylase 1 |
|---|
| Name: | Histone deacetylase 1 |
|---|
| Synonyms: | HD1 | HDAC1_MOUSE | Hdac1 | Histone deacetylase 1/2 |
|---|
| Type: | Protein |
|---|
| Mol. Mass.: | 55062.26 |
|---|
| Organism: | Mus musculus (Mouse) |
|---|
| Description: | O09106 |
|---|
| Residue: | 482 |
|---|
| Sequence: | MAQTQGTKRKVCYYYDGDVGNYYYGQGHPMKPHRIRMTHNLLLNYGLYRKMEIYRPHKAN
AEEMTKYHSDDYIKFLRSIRPDNMSEYSKQMQRFNVGEDCPVFDGLFEFCQLSTGGSVAS
AVKLNKQQTDIAVNWAGGLHHAKKSEASGFCYVNDIVLAILELLKYHQRVLYIDIDIHHG
DGVEEAFYTTDRVMTVSFHKYGEYFPGTGDLRDIGAGKGKYYAVNYPLRDGIDDESYEAI
FKPVMSKVMEMFQPSAVVLQCGSDSLSGDRLGCFNLTIKGHAKCVEFVKSFNLPMLMLGG
GGYTIRNVARCWTYETAVALDTEIPNELPYNDYFEYFGPDFKLHISPSNMTNQNTNEYLE
KIKQRLFENLRMLPHAPGVQMQAIPEDAIPEESGDEDEEDPDKRISICSSDKRIACEEEF
SDSDEEGEGGRKNSSNFKKAKRVKTEDEKEKDPEEKKEVTEEEKTKEEKPEAKGVKEEVK
LA
|
|
|
|---|
| BDBM50100456 |
|---|
| n/a |
|---|
| Name | BDBM50100456 |
|---|
| Synonyms: | CHEMBL51356 | N-Hydroxy-3-[(E)-1-methyl-4-(4-methyl-benzoyl)-1H-pyrrol-2-yl]-acrylamide | N-Hydroxy-3-[1-methyl-4-(4-methyl-benzoyl)-1H-pyrrol-2-yl]-acrylamide |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C16H16N2O3 |
|---|
| Mol. Mass. | 284.3098 |
|---|
| SMILES | Cc1ccc(cc1)C(=O)c1cc(\C=C\C(=O)NO)n(C)c1 |
|---|
| Structure | 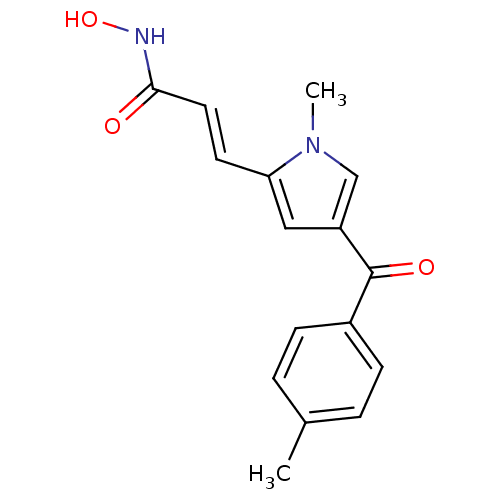
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Mai, A; Massa, S; Ragno, R; Esposito, M; Sbardella, G; Nocca, G; Scatena, R; Jesacher, F; Loidl, P; Brosch, G Binding mode analysis of 3-(4-benzoyl-1-methyl-1H-2-pyrrolyl)-N-hydroxy-2-propenamide: a new synthetic histone deacetylase inhibitor inducing histone hyperacetylation, growth inhibition, and terminal cell differentiation. J Med Chem45:1778-84 (2002) [PubMed]
Mai, A; Massa, S; Ragno, R; Esposito, M; Sbardella, G; Nocca, G; Scatena, R; Jesacher, F; Loidl, P; Brosch, G Binding mode analysis of 3-(4-benzoyl-1-methyl-1H-2-pyrrolyl)-N-hydroxy-2-propenamide: a new synthetic histone deacetylase inhibitor inducing histone hyperacetylation, growth inhibition, and terminal cell differentiation. J Med Chem45:1778-84 (2002) [PubMed]