| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Cytochrome P450 2A6 |
|---|
| Ligand | BDBM50555408 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_2051941 (CHEMBL4706942) |
|---|
| IC50 | >10000±n/a nM |
|---|
| Citation |  Osborne, JD; Matthews, TP; McHardy, T; Proisy, N; Cheung, KM; Lainchbury, M; Brown, N; Walton, MI; Eve, PD; Boxall, KJ; Hayes, A; Henley, AT; Valenti, MR; De Haven Brandon, AK; Box, G; Jamin, Y; Robinson, SP; Westwood, IM; van Montfort, RL; Leonard, PM; Lamers, MB; Reader, JC; Aherne, GW; Raynaud, FI; Eccles, SA; Garrett, MD; Collins, I Multiparameter Lead Optimization to Give an Oral Checkpoint Kinase 1 (CHK1) Inhibitor Clinical Candidate: (R)-5-((4-((Morpholin-2-ylmethyl)amino)-5-(trifluoromethyl)pyridin-2-yl)amino)pyrazine-2-carbonitrile (CCT245737). J Med Chem59:5221-37 (2016) [PubMed] Article Osborne, JD; Matthews, TP; McHardy, T; Proisy, N; Cheung, KM; Lainchbury, M; Brown, N; Walton, MI; Eve, PD; Boxall, KJ; Hayes, A; Henley, AT; Valenti, MR; De Haven Brandon, AK; Box, G; Jamin, Y; Robinson, SP; Westwood, IM; van Montfort, RL; Leonard, PM; Lamers, MB; Reader, JC; Aherne, GW; Raynaud, FI; Eccles, SA; Garrett, MD; Collins, I Multiparameter Lead Optimization to Give an Oral Checkpoint Kinase 1 (CHK1) Inhibitor Clinical Candidate: (R)-5-((4-((Morpholin-2-ylmethyl)amino)-5-(trifluoromethyl)pyridin-2-yl)amino)pyrazine-2-carbonitrile (CCT245737). J Med Chem59:5221-37 (2016) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Cytochrome P450 2A6 |
|---|
| Name: | Cytochrome P450 2A6 |
|---|
| Synonyms: | 1,4-cineole 2-exo-monooxygenase | 1.14.13.- | CP2A6_HUMAN | CYP2A3 | CYP2A6 | CYPIIA6 | Coumarin 7-hydroxylase | Cytochrome P450 2A6 | Cytochrome P450 IIA3 | Cytochrome P450(I) |
|---|
| Type: | Protein |
|---|
| Mol. Mass.: | 56514.34 |
|---|
| Organism: | Homo sapiens (Human) |
|---|
| Description: | P11509 |
|---|
| Residue: | 494 |
|---|
| Sequence: | MLASGMLLVALLVCLTVMVLMSVWQQRKSKGKLPPGPTPLPFIGNYLQLNTEQMYNSLMK
ISERYGPVFTIHLGPRRVVVLCGHDAVREALVDQAEEFSGRGEQATFDWVFKGYGVVFSN
GERAKQLRRFSIATLRDFGVGKRGIEERIQEEAGFLIDALRGTGGANIDPTFFLSRTVSN
VISSIVFGDRFDYKDKEFLSLLRMMLGIFQFTSTSTGQLYEMFSSVMKHLPGPQQQAFQL
LQGLEDFIAKKVEHNQRTLDPNSPRDFIDSFLIRMQEEEKNPNTEFYLKNLVMTTLNLFI
GGTETVSTTLRYGFLLLMKHPEVEAKVHEEIDRVIGKNRQPKFEDRAKMPYMEAVIHEIQ
RFGDVIPMSLARRVKKDTKFRDFFLPKGTEVYPMLGSVLRDPSFFSNPQDFNPQHFLNEK
GQFKKSDAFVPFSIGKRNCFGEGLARMELFLFFTTVMQNFRLKSSQSPKDIDVSPKHVGF
ATIPRNYTMSFLPR
|
|
|
|---|
| BDBM50555408 |
|---|
| n/a |
|---|
| Name | BDBM50555408 |
|---|
| Synonyms: | CHEMBL4792322 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C18H21N7O |
|---|
| Mol. Mass. | 351.4056 |
|---|
| SMILES | N#Cc1cnc(Nc2cc(NC[C@@H]3CNCCO3)c(cn2)C2CC2)cn1 |r| |
|---|
| Structure | 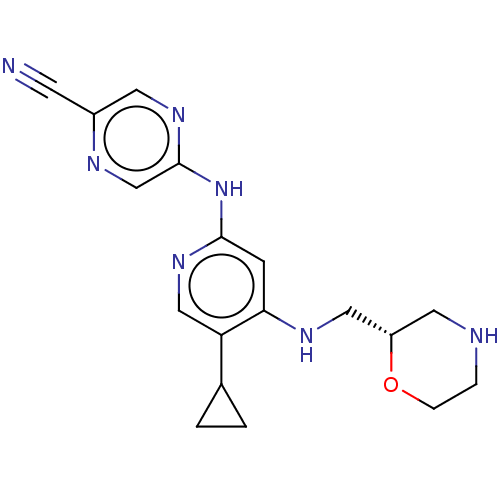
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Osborne, JD; Matthews, TP; McHardy, T; Proisy, N; Cheung, KM; Lainchbury, M; Brown, N; Walton, MI; Eve, PD; Boxall, KJ; Hayes, A; Henley, AT; Valenti, MR; De Haven Brandon, AK; Box, G; Jamin, Y; Robinson, SP; Westwood, IM; van Montfort, RL; Leonard, PM; Lamers, MB; Reader, JC; Aherne, GW; Raynaud, FI; Eccles, SA; Garrett, MD; Collins, I Multiparameter Lead Optimization to Give an Oral Checkpoint Kinase 1 (CHK1) Inhibitor Clinical Candidate: (R)-5-((4-((Morpholin-2-ylmethyl)amino)-5-(trifluoromethyl)pyridin-2-yl)amino)pyrazine-2-carbonitrile (CCT245737). J Med Chem59:5221-37 (2016) [PubMed] Article
Osborne, JD; Matthews, TP; McHardy, T; Proisy, N; Cheung, KM; Lainchbury, M; Brown, N; Walton, MI; Eve, PD; Boxall, KJ; Hayes, A; Henley, AT; Valenti, MR; De Haven Brandon, AK; Box, G; Jamin, Y; Robinson, SP; Westwood, IM; van Montfort, RL; Leonard, PM; Lamers, MB; Reader, JC; Aherne, GW; Raynaud, FI; Eccles, SA; Garrett, MD; Collins, I Multiparameter Lead Optimization to Give an Oral Checkpoint Kinase 1 (CHK1) Inhibitor Clinical Candidate: (R)-5-((4-((Morpholin-2-ylmethyl)amino)-5-(trifluoromethyl)pyridin-2-yl)amino)pyrazine-2-carbonitrile (CCT245737). J Med Chem59:5221-37 (2016) [PubMed] Article