| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Beta-galactosidase |
|---|
| Ligand | BDBM50403937 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_39391 (CHEMBL659249) |
|---|
| IC50 | 65000±n/a nM |
|---|
| Citation |  Moreno-Vargas, AJ; Demange, R; Fuentes, J; Robina, I; Vogel, P Synthesis of [(2S,3S,4R)-3,4-dihydroxypyrrolidin-2-yl]-5-methylfuran-4-carboxylic acid derivatives: new leads as selective beta-galactosidase inhibitors. Bioorg Med Chem Lett12:2335-9 (2002) [PubMed] Moreno-Vargas, AJ; Demange, R; Fuentes, J; Robina, I; Vogel, P Synthesis of [(2S,3S,4R)-3,4-dihydroxypyrrolidin-2-yl]-5-methylfuran-4-carboxylic acid derivatives: new leads as selective beta-galactosidase inhibitors. Bioorg Med Chem Lett12:2335-9 (2002) [PubMed] |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Beta-galactosidase |
|---|
| Name: | Beta-galactosidase |
|---|
| Synonyms: | Acid beta-galactosidase | BGAL_HUMAN | ELNR1 | Elastin receptor 1 | GLB1 | Lactase |
|---|
| Type: | PROTEIN |
|---|
| Mol. Mass.: | 76074.43 |
|---|
| Organism: | Homo sapiens (Human) |
|---|
| Description: | ChEMBL_215886 |
|---|
| Residue: | 677 |
|---|
| Sequence: | MPGFLVRILPLLLVLLLLGPTRGLRNATQRMFEIDYSRDSFLKDGQPFRYISGSIHYSRV
PRFYWKDRLLKMKMAGLNAIQTYVPWNFHEPWPGQYQFSEDHDVEYFLRLAHELGLLVIL
RPGPYICAEWEMGGLPAWLLEKESILLRSSDPDYLAAVDKWLGVLLPKMKPLLYQNGGPV
ITVQVENEYGSYFACDFDYLRFLQKRFRHHLGDDVVLFTTDGAHKTFLKCGALQGLYTTV
DFGTGSNITDAFLSQRKCEPKGPLINSEFYTGWLDHWGQPHSTIKTEAVASSLYDILARG
ASVNLYMFIGGTNFAYWNGANSPYAAQPTSYDYDAPLSEAGDLTEKYFALRNIIQKFEKV
PEGPIPPSTPKFAYGKVTLEKLKTVGAALDILCPSGPIKSLYPLTFIQVKQHYGFVLYRT
TLPQDCSNPAPLSSPLNGVHDRAYVAVDGIPQGVLERNNVITLNITGKAGATLDLLVENM
GRVNYGAYINDFKGLVSNLTLSSNILTDWTIFPLDTEDAVRSHLGGWGHRDSGHHDEAWA
HNSSNYTLPAFYMGNFSIPSGIPDLPQDTFIQFPGWTKGQVWINGFNLGRYWPARGPQLT
LFVPQHILMTSAPNTITVLELEWAPCSSDDPELCAVTFVDRPVIGSSVTYDHPSKPVEKR
LMPPPPQKNKDSWLDHV
|
|
|
|---|
| BDBM50403937 |
|---|
| n/a |
|---|
| Name | BDBM50403937 |
|---|
| Synonyms: | CHEMBL2114149 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C14H22N2O4 |
|---|
| Mol. Mass. | 282.3355 |
|---|
| SMILES | CCN(CC)C(=O)c1cc(oc1C)[C@H]1NC[C@@H](O)[C@H]1O |r| |
|---|
| Structure | 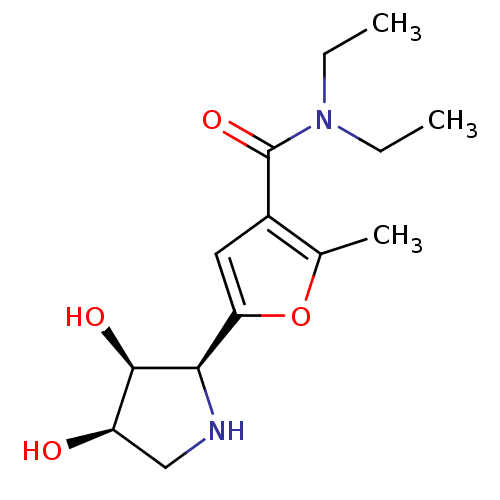
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Moreno-Vargas, AJ; Demange, R; Fuentes, J; Robina, I; Vogel, P Synthesis of [(2S,3S,4R)-3,4-dihydroxypyrrolidin-2-yl]-5-methylfuran-4-carboxylic acid derivatives: new leads as selective beta-galactosidase inhibitors. Bioorg Med Chem Lett12:2335-9 (2002) [PubMed]
Moreno-Vargas, AJ; Demange, R; Fuentes, J; Robina, I; Vogel, P Synthesis of [(2S,3S,4R)-3,4-dihydroxypyrrolidin-2-yl]-5-methylfuran-4-carboxylic acid derivatives: new leads as selective beta-galactosidase inhibitors. Bioorg Med Chem Lett12:2335-9 (2002) [PubMed]