| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Chymotrypsin-C |
|---|
| Ligand | BDBM50120286 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_197808 (CHEMBL802338) |
|---|
| IC50 | 280±n/a nM |
|---|
| Citation |  Priestley, ES; De Lucca, I; Ghavimi, B; Erickson-Viitanen, S; Decicco, CP P1 Phenethyl peptide boronic acid inhibitors of HCV NS3 protease. Bioorg Med Chem Lett12:3199-202 (2002) [PubMed] Priestley, ES; De Lucca, I; Ghavimi, B; Erickson-Viitanen, S; Decicco, CP P1 Phenethyl peptide boronic acid inhibitors of HCV NS3 protease. Bioorg Med Chem Lett12:3199-202 (2002) [PubMed] |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Chymotrypsin-C |
|---|
| Name: | Chymotrypsin-C |
|---|
| Synonyms: | CLCR | CTRC | CTRC_HUMAN | Caldecrin | Chymotrypsin | Chymotrypsin C | Chymotrypsin-C |
|---|
| Type: | Enzyme |
|---|
| Mol. Mass.: | 29487.98 |
|---|
| Organism: | Homo sapiens (Human) |
|---|
| Description: | Q99895 |
|---|
| Residue: | 268 |
|---|
| Sequence: | MLGITVLAALLACASSCGVPSFPPNLSARVVGGEDARPHSWPWQISLQYLKNDTWRHTCG
GTLIASNFVLTAAHCISNTRTYRVAVGKNNLEVEDEEGSLFVGVDTIHVHKRWNALLLRN
DIALIKLAEHVELSDTIQVACLPEKDSLLPKDYPCYVTGWGRLWTNGPIADKLQQGLQPV
VDHATCSRIDWWGFRVKKTMVCAGGDGVISACNGDSGGPLNCQLENGSWEVFGIVSFGSR
RGCNTRKKPVVYTRVSAYIDWINEKMQL
|
|
|
|---|
| BDBM50120286 |
|---|
| n/a |
|---|
| Name | BDBM50120286 |
|---|
| Synonyms: | CHEMBL322933 | Peptide Boronic Acid analogue |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C41H69BN6O11 |
|---|
| Mol. Mass. | 832.831 |
|---|
| SMILES | CC(C)CCC[C@H](NC(=O)[C@H]1CCCN1C(=O)[C@@H](NC(=O)[C@@H](NC(=O)[C@H](CCC(O)=O)NC(=O)[C@@H](N)CC(O)=O)C(C)C)C(C)C)B1O[C@@H]2C[C@@H]3C[C@@H](C3(C)C)[C@]2(C)O1 |
|---|
| Structure | 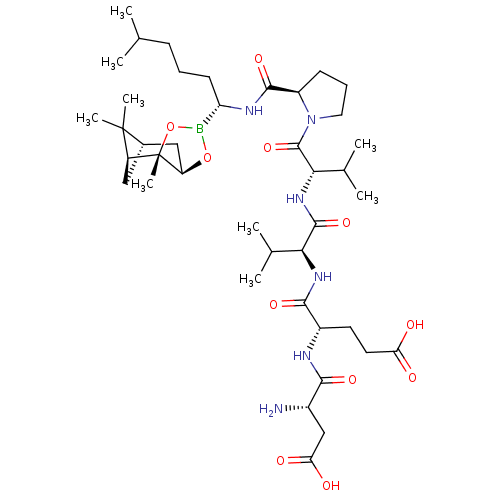
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Priestley, ES; De Lucca, I; Ghavimi, B; Erickson-Viitanen, S; Decicco, CP P1 Phenethyl peptide boronic acid inhibitors of HCV NS3 protease. Bioorg Med Chem Lett12:3199-202 (2002) [PubMed]
Priestley, ES; De Lucca, I; Ghavimi, B; Erickson-Viitanen, S; Decicco, CP P1 Phenethyl peptide boronic acid inhibitors of HCV NS3 protease. Bioorg Med Chem Lett12:3199-202 (2002) [PubMed]