| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Tripeptidyl-peptidase 2 |
|---|
| Ligand | BDBM50121282 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_211946 (CHEMBL816536) |
|---|
| IC50 | 7±n/a nM |
|---|
| Citation |  Breslin, HJ; Miskowski, TA; Kukla, MJ; Leister, WH; De Winter, HL; Gauthier, DA; Somers, MV; Peeters, DC; Roevens, PW Design, synthesis, and tripeptidyl peptidase II inhibitory activity of a novel series of (S)-2,3-dihydro-2-(4-alkyl-1H-imidazol-2-yl)-1H-indoles. J Med Chem45:5303-10 (2002) [PubMed] Breslin, HJ; Miskowski, TA; Kukla, MJ; Leister, WH; De Winter, HL; Gauthier, DA; Somers, MV; Peeters, DC; Roevens, PW Design, synthesis, and tripeptidyl peptidase II inhibitory activity of a novel series of (S)-2,3-dihydro-2-(4-alkyl-1H-imidazol-2-yl)-1H-indoles. J Med Chem45:5303-10 (2002) [PubMed] |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Tripeptidyl-peptidase 2 |
|---|
| Name: | Tripeptidyl-peptidase 2 |
|---|
| Synonyms: | TPP2_RAT | Tpp2 | Tripeptidyl aminopeptidase |
|---|
| Type: | PROTEIN |
|---|
| Mol. Mass.: | 138287.08 |
|---|
| Organism: | Rattus norvegicus |
|---|
| Description: | ChEMBL_211948 |
|---|
| Residue: | 1249 |
|---|
| Sequence: | MATAATEEPFPFHGLLPKKETGASSFLCRYPEYDGRGVLIAVLDTGVDPGAPGMQVTTDG
KPKIIDIIDTTGSGDVNTATEVEPKDGEITGLSGRVLKIPANWTNPSGKYHIGIKNGYDF
YPKALKERIQKERKEKIWDPIHRVALAEACRKQEEFDIANNGSSQANKLIKEELQSQVEL
LNSFEKKYSDPGPVYDCLVWHDGETWRACVDSNENGDLGKSTVLRNYKEAQEYGSFGTAE
MLNYSVNIYDDGNLLSIVTSGGAHGTHVASIAAGHFPEEPERNGVAPGAQILSIKIGDTR
LSTMETGTGLIRAMIEVINHKCDLVNYSYGEATHWPNSGRICEVINEAVWKHNTIYVSSA
GNNGPCLSTVGCPGGTTSSVIGVGAYVSPDMMVAEYSLREKLPANQYTWSSRGPSADGAL
GVSISAPGGAIASVPNWTLRGTQLMNGTSMSSPNACGGIALVLSGLKANNVDYTVHSVRR
ALENTAIKADNIEVFAQGHGIIQVDKAYDYLIQNTSFANRLGFTVTVGNNRGIYLRDPVQ
VAAPSDHGVGIEPVFPENTENSEKISFQLHLALTSNSSWVQCPSHLELMNQCRHINIRVD
PRGLREGLHYTEVCGYDIASPNAGPLFRVPITAVIAAKVNESSHYDLAFTDVHFKPGQIR
RHFVEVPEGATWAEVTVCSCSSEVSAKFVLHAVQLVKQRAYRSHEFYKFCSLPEKGTLIE
AFPVLGGKAIEFCIARWWASLSDVNIDYTISFHGIVCTAPQLNIHASEGINRFDVQSSLK
YEDLAPCITLKSWVQTLRPVNAKTRPLGSRDVLPNNRQLYEMVLTYSFHQPKSGEVTPSC
PLLCELLYESEFDSQLWIIFDQNKRQMGSGDAYPHQYSLKLEKGDYTIRLQIRHEQISDL
DRLKDLPFIVSHRLSNTLSLDIHENHSLALLGKKKSSSLTLPPKYNQPFFVTSLPDDKIP
KGAGPGCYLAGSLTLSKTELGKKADVIPVHYYLIPPPTKTKNGSKDKEKDSEKEKDLKEE
FTEALRDLKIQWMTKLDSTDIYNELKETYPAYLPLYVARLHQLDAEKERMKRLNEIVDAA
NAVISHIDQTALAVYIAMKTDPRPDAATIKNDMDKQKSTLVDALCRKGCALADHLLHAQP
HDGAAAGDAEAKEEEGESTLESLSETYWETTKWTDLFDTKVLTFAYKHALVNKMYGRGLK
FATKLVEEKPTKENWKNCIQLMKLLGWTHCASFTENWLPIMYPPDYCVF
|
|
|
|---|
| BDBM50121282 |
|---|
| n/a |
|---|
| Name | BDBM50121282 |
|---|
| Synonyms: | (S)-1-((S)-2-aminobutanoyl)-N-butylindoline-2-carboxamide | 1-(2-Amino-butyryl)-2,3-dihydro-1H-indole-2-carboxylic acid butylamide | CHEMBL151059 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C17H25N3O2 |
|---|
| Mol. Mass. | 303.3993 |
|---|
| SMILES | CCCCNC(=O)[C@@H]1Cc2ccccc2N1C(=O)[C@@H](N)CC |
|---|
| Structure | 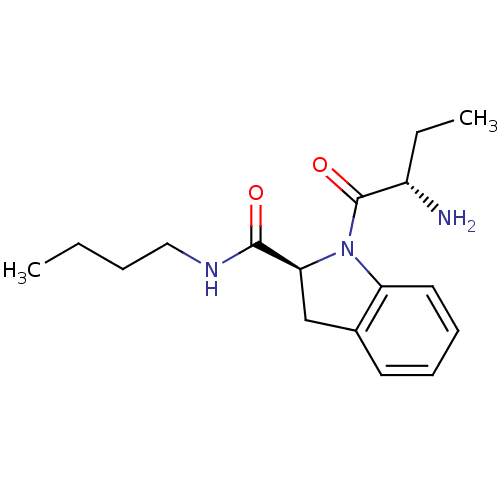
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Breslin, HJ; Miskowski, TA; Kukla, MJ; Leister, WH; De Winter, HL; Gauthier, DA; Somers, MV; Peeters, DC; Roevens, PW Design, synthesis, and tripeptidyl peptidase II inhibitory activity of a novel series of (S)-2,3-dihydro-2-(4-alkyl-1H-imidazol-2-yl)-1H-indoles. J Med Chem45:5303-10 (2002) [PubMed]
Breslin, HJ; Miskowski, TA; Kukla, MJ; Leister, WH; De Winter, HL; Gauthier, DA; Somers, MV; Peeters, DC; Roevens, PW Design, synthesis, and tripeptidyl peptidase II inhibitory activity of a novel series of (S)-2,3-dihydro-2-(4-alkyl-1H-imidazol-2-yl)-1H-indoles. J Med Chem45:5303-10 (2002) [PubMed]