| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Lactoylglutathione lyase |
|---|
| Ligand | BDBM50343659 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_2071491 (CHEMBL4727025) |
|---|
| IC50 | 4450±n/a nM |
|---|
| Citation |  Al-Oudat, BA; Jaradat, HM; Al-Balas, QA; Al-Shar'i, NA; Bryant-Friedrich, A; Bedi, MF Design, synthesis and biological evaluation of novel glyoxalase I inhibitors possessing diazenylbenzenesulfonamide moiety as potential anticancer agents. Bioorg Med Chem28:0 (2020) [PubMed] Article Al-Oudat, BA; Jaradat, HM; Al-Balas, QA; Al-Shar'i, NA; Bryant-Friedrich, A; Bedi, MF Design, synthesis and biological evaluation of novel glyoxalase I inhibitors possessing diazenylbenzenesulfonamide moiety as potential anticancer agents. Bioorg Med Chem28:0 (2020) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Lactoylglutathione lyase |
|---|
| Name: | Lactoylglutathione lyase |
|---|
| Synonyms: | Aldoketomutase | GLO1 | Glx I | Glyoxalase 1 (GLO1) | Glyoxalase I | Ketone-aldehyde mutase | LGUL_HUMAN | Methylglyoxalase | S-D-lactoylglutathione methylglyoxal lyase |
|---|
| Type: | Enzyme |
|---|
| Mol. Mass.: | 20772.95 |
|---|
| Organism: | Homo sapiens (Human) |
|---|
| Description: | Q04760 |
|---|
| Residue: | 184 |
|---|
| Sequence: | MAEPQPPSGGLTDEAALSCCSDADPSTKDFLLQQTMLRVKDPKKSLDFYTRVLGMTLIQK
CDFPIMKFSLYFLAYEDKNDIPKEKDEKIAWALSRKATLELTHNWGTEDDETQSYHNGNS
DPRGFGHIGIAVPDVYSACKRFEELGVKFVKKPDDGKMKGLAFIQDPDGYWIEILNPNKM
ATLM
|
|
|
|---|
| BDBM50343659 |
|---|
| n/a |
|---|
| Name | BDBM50343659 |
|---|
| Synonyms: | (E)-4-((8-hydroxyquinolin-5-yl)diazenyl)benzoic acid | CHEMBL1775074 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C16H11N3O3 |
|---|
| Mol. Mass. | 293.2768 |
|---|
| SMILES | OC(=O)c1ccc(cc1)N=Nc1ccc(O)c2ncccc12 |w:9.9| |
|---|
| Structure | 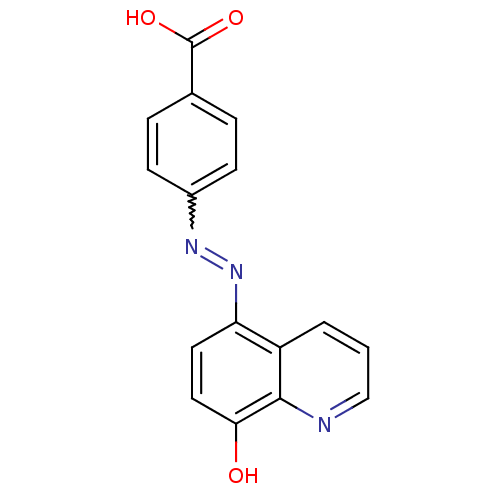
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Al-Oudat, BA; Jaradat, HM; Al-Balas, QA; Al-Shar'i, NA; Bryant-Friedrich, A; Bedi, MF Design, synthesis and biological evaluation of novel glyoxalase I inhibitors possessing diazenylbenzenesulfonamide moiety as potential anticancer agents. Bioorg Med Chem28:0 (2020) [PubMed] Article
Al-Oudat, BA; Jaradat, HM; Al-Balas, QA; Al-Shar'i, NA; Bryant-Friedrich, A; Bedi, MF Design, synthesis and biological evaluation of novel glyoxalase I inhibitors possessing diazenylbenzenesulfonamide moiety as potential anticancer agents. Bioorg Med Chem28:0 (2020) [PubMed] Article