| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Histone deacetylase 1 |
|---|
| Ligand | BDBM50105692 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_87856 (CHEMBL877713) |
|---|
| IC50 | 100±n/a nM |
|---|
| Citation |  Bouchain, G; Leit, S; Frechette, S; Khalil, EA; Lavoie, R; Moradei, O; Woo, SH; Fournel, M; Yan, PT; Kalita, A; Trachy-Bourget, MC; Beaulieu, C; Li, Z; Robert, MF; MacLeod, AR; Besterman, JM; Delorme, D Development of potential antitumor agents. Synthesis and biological evaluation of a new set of sulfonamide derivatives as histone deacetylase inhibitors. J Med Chem46:820-30 (2003) [PubMed] Article Bouchain, G; Leit, S; Frechette, S; Khalil, EA; Lavoie, R; Moradei, O; Woo, SH; Fournel, M; Yan, PT; Kalita, A; Trachy-Bourget, MC; Beaulieu, C; Li, Z; Robert, MF; MacLeod, AR; Besterman, JM; Delorme, D Development of potential antitumor agents. Synthesis and biological evaluation of a new set of sulfonamide derivatives as histone deacetylase inhibitors. J Med Chem46:820-30 (2003) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Histone deacetylase 1 |
|---|
| Name: | Histone deacetylase 1 |
|---|
| Synonyms: | Cereblon/Histone deacetylase 1 | HD1 | HDAC1 | HDAC1_HUMAN | Histone deacetylase 1 (HDAC1) | Human HDAC1 | RPD3L1 |
|---|
| Type: | Enzyme |
|---|
| Mol. Mass.: | 55090.27 |
|---|
| Organism: | Homo sapiens (Human) |
|---|
| Description: | Q13547 |
|---|
| Residue: | 482 |
|---|
| Sequence: | MAQTQGTRRKVCYYYDGDVGNYYYGQGHPMKPHRIRMTHNLLLNYGLYRKMEIYRPHKAN
AEEMTKYHSDDYIKFLRSIRPDNMSEYSKQMQRFNVGEDCPVFDGLFEFCQLSTGGSVAS
AVKLNKQQTDIAVNWAGGLHHAKKSEASGFCYVNDIVLAILELLKYHQRVLYIDIDIHHG
DGVEEAFYTTDRVMTVSFHKYGEYFPGTGDLRDIGAGKGKYYAVNYPLRDGIDDESYEAI
FKPVMSKVMEMFQPSAVVLQCGSDSLSGDRLGCFNLTIKGHAKCVEFVKSFNLPMLMLGG
GGYTIRNVARCWTYETAVALDTEIPNELPYNDYFEYFGPDFKLHISPSNMTNQNTNEYLE
KIKQRLFENLRMLPHAPGVQMQAIPEDAIPEESGDEDEDDPDKRISICSSDKRIACEEEF
SDSEEEGEGGRKNSSNFKKAKRVKTEDEKEKDPEEKKEVTEEEKTKEEKPEAKGVKEEVK
LA
|
|
|
|---|
| BDBM50105692 |
|---|
| n/a |
|---|
| Name | BDBM50105692 |
|---|
| Synonyms: | (E)-3-[4-(4-tert-Butyl-benzenesulfonylamino)-phenyl]-N-hydroxy-acrylamide | 3-(4-(4-tert-butylphenylsulfonamido)phenyl)-N-hydroxyacrylamide | 3-[4-(4-tert-Butyl-benzenesulfonylamino)-phenyl]-N-hydroxy-acrylamide | CHEMBL97272 | US8796330, 105 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C19H22N2O4S |
|---|
| Mol. Mass. | 374.454 |
|---|
| SMILES | CC(C)(C)c1ccc(cc1)S(=O)(=O)Nc1ccc(\C=C\C(=O)NO)cc1 |
|---|
| Structure | 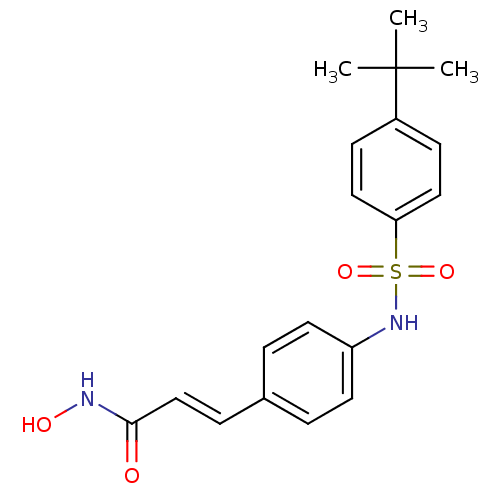
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Bouchain, G; Leit, S; Frechette, S; Khalil, EA; Lavoie, R; Moradei, O; Woo, SH; Fournel, M; Yan, PT; Kalita, A; Trachy-Bourget, MC; Beaulieu, C; Li, Z; Robert, MF; MacLeod, AR; Besterman, JM; Delorme, D Development of potential antitumor agents. Synthesis and biological evaluation of a new set of sulfonamide derivatives as histone deacetylase inhibitors. J Med Chem46:820-30 (2003) [PubMed] Article
Bouchain, G; Leit, S; Frechette, S; Khalil, EA; Lavoie, R; Moradei, O; Woo, SH; Fournel, M; Yan, PT; Kalita, A; Trachy-Bourget, MC; Beaulieu, C; Li, Z; Robert, MF; MacLeod, AR; Besterman, JM; Delorme, D Development of potential antitumor agents. Synthesis and biological evaluation of a new set of sulfonamide derivatives as histone deacetylase inhibitors. J Med Chem46:820-30 (2003) [PubMed] Article