| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Peroxisome proliferator-activated receptor gamma |
|---|
| Ligand | BDBM50127920 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_154547 (CHEMBL757882) |
|---|
| EC50 | 80±n/a nM |
|---|
| Citation |  Koyama, H; Boueres, JK; Han, W; Metzger, EJ; Bergman, JP; Gratale, DF; Miller, DJ; Tolman, RL; MacNaul, KL; Berger, JP; Doebber, TW; Leung, K; Moller, DE; Heck, JV; Sahoo, SP 5-Aryl thiazolidine-2,4-diones as selective PPARgamma agonists. Bioorg Med Chem Lett13:1801-4 (2003) [PubMed] Koyama, H; Boueres, JK; Han, W; Metzger, EJ; Bergman, JP; Gratale, DF; Miller, DJ; Tolman, RL; MacNaul, KL; Berger, JP; Doebber, TW; Leung, K; Moller, DE; Heck, JV; Sahoo, SP 5-Aryl thiazolidine-2,4-diones as selective PPARgamma agonists. Bioorg Med Chem Lett13:1801-4 (2003) [PubMed] |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Peroxisome proliferator-activated receptor gamma |
|---|
| Name: | Peroxisome proliferator-activated receptor gamma |
|---|
| Synonyms: | NR1C3 | Nuclear receptor subfamily 1 group C member 3 | PPAR-gamma | PPARG | PPARG_HUMAN | Peroxisome proliferator-activated receptor | Peroxisome proliferator-activated receptor gamma (PPAR gamma) | Peroxisome proliferator-activated receptor gamma (PPARG) | Peroxisome proliferator-activated receptor gamma (PPARγ) | Peroxisome proliferator-activated receptor gamma/Nuclear receptor corepressor 2 | peroxisome proliferator-activated receptor gamma isoform 2 |
|---|
| Type: | Nuclear Receptor |
|---|
| Mol. Mass.: | 57613.46 |
|---|
| Organism: | Homo sapiens (Human) |
|---|
| Description: | P37231 |
|---|
| Residue: | 505 |
|---|
| Sequence: | MGETLGDSPIDPESDSFTDTLSANISQEMTMVDTEMPFWPTNFGISSVDLSVMEDHSHSF
DIKPFTTVDFSSISTPHYEDIPFTRTDPVVADYKYDLKLQEYQSAIKVEPASPPYYSEKT
QLYNKPHEEPSNSLMAIECRVCGDKASGFHYGVHACEGCKGFFRRTIRLKLIYDRCDLNC
RIHKKSRNKCQYCRFQKCLAVGMSHNAIRFGRMPQAEKEKLLAEISSDIDQLNPESADLR
ALAKHLYDSYIKSFPLTKAKARAILTGKTTDKSPFVIYDMNSLMMGEDKIKFKHITPLQE
QSKEVAIRIFQGCQFRSVEAVQEITEYAKSIPGFVNLDLNDQVTLLKYGVHEIIYTMLAS
LMNKDGVLISEGQGFMTREFLKSLRKPFGDFMEPKFEFAVKFNALELDDSDLAIFIAVII
LSGDRPGLLNVKPIEDIQDNLLQALELQLKLNHPESSQLFAKLLQKMTDLRQIVTEHVQL
LQVIKKTETDMSLHPLLQEIYKDLY
|
|
|
|---|
| BDBM50127920 |
|---|
| n/a |
|---|
| Name | BDBM50127920 |
|---|
| Synonyms: | 5-(4-{3-[4-(4-Chloro-phenoxy)-2-propyl-phenoxy]-propoxy}-phenyl)-thiazolidine-2,4-dione | CHEMBL52426 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C27H26ClNO5S |
|---|
| Mol. Mass. | 512.017 |
|---|
| SMILES | CCCc1cc(Oc2ccc(Cl)cc2)ccc1OCCCOc1ccc(cc1)-c1sc(=O)[nH]c1O |
|---|
| Structure | 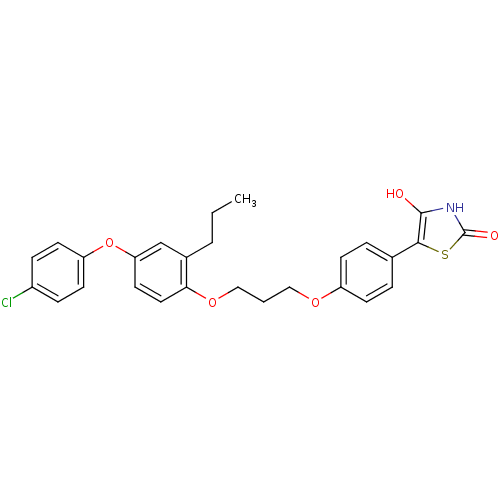
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Koyama, H; Boueres, JK; Han, W; Metzger, EJ; Bergman, JP; Gratale, DF; Miller, DJ; Tolman, RL; MacNaul, KL; Berger, JP; Doebber, TW; Leung, K; Moller, DE; Heck, JV; Sahoo, SP 5-Aryl thiazolidine-2,4-diones as selective PPARgamma agonists. Bioorg Med Chem Lett13:1801-4 (2003) [PubMed]
Koyama, H; Boueres, JK; Han, W; Metzger, EJ; Bergman, JP; Gratale, DF; Miller, DJ; Tolman, RL; MacNaul, KL; Berger, JP; Doebber, TW; Leung, K; Moller, DE; Heck, JV; Sahoo, SP 5-Aryl thiazolidine-2,4-diones as selective PPARgamma agonists. Bioorg Med Chem Lett13:1801-4 (2003) [PubMed]