| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Prolyl endopeptidase |
|---|
| Ligand | BDBM50134158 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_223468 (CHEMBL874055) |
|---|
| IC50 | 87±n/a nM |
|---|
| Citation |  Wallén, EA; Christiaans, JA; Jarho, EM; Forsberg, MM; Venäläinen, JI; Männistö, PT; Gynther, J New prolyl oligopeptidase inhibitors developed from dicarboxylic acid bis(l-prolyl-pyrrolidine) amides. J Med Chem46:4543-51 (2003) [PubMed] Article Wallén, EA; Christiaans, JA; Jarho, EM; Forsberg, MM; Venäläinen, JI; Männistö, PT; Gynther, J New prolyl oligopeptidase inhibitors developed from dicarboxylic acid bis(l-prolyl-pyrrolidine) amides. J Med Chem46:4543-51 (2003) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Prolyl endopeptidase |
|---|
| Name: | Prolyl endopeptidase |
|---|
| Synonyms: | 3.4.21.26 | PE | PPCE_PIG | PREP | Post-proline cleaving enzyme |
|---|
| Type: | n/a |
|---|
| Mol. Mass.: | 80758.04 |
|---|
| Organism: | Sus scrofa |
|---|
| Description: | n/a |
|---|
| Residue: | 710 |
|---|
| Sequence: | MLSFQYPDVYRDETAIQDYHGHKVCDPYAWLEDPDSEQTKAFVEAQNKITVPFLEQCPIR
GLYKERMTELYDYPKYSCHFKKGKRYFYFYNTGLQNQRVLYVQDSLEGEARVFLDPNILS
DDGTVALRGYAFSEDGEYFAYGLSASGSDWVTIKFMKVDGAKELPDVLERVKFSCMAWTH
DGKGMFYNAYPQQDGKSDGTETSTNLHQKLYYHVLGTDQSEDILCAEFPDEPKWMGGAEL
SDDGRYVLLSIREGCDPVNRLWYCDLQQESNGITGILKWVKLIDNFEGEYDYVTNEGTVF
TFKTNRHSPNYRLINIDFTDPEESKWKVLVPEHEKDVLEWVACVRSNFLVLCYLHDVKNT
LQLHDLATGALLKIFPLEVGSVVGYSGQKKDTEIFYQFTSFLSPGIIYHCDLTKEELEPR
VFREVTVKGIDASDYQTVQIFYPSKDGTKIPMFIVHKKGIKLDGSHPAFLYGYGGFNISI
TPNYSVSRLIFVRHMGGVLAVANIRGGGEYGETWHKGGILANKQNCFDDFQCAAEYLIKE
GYTSPKRLTINGGSNGGLLVATCANQRPDLFGCVIAQVGVMDMLKFHKYTIGHAWTTDYG
CSDSKQHFEWLIKYSPLHNVKLPEADDIQYPSMLLLTADHDDRVVPLHSLKFIATLQYIV
GRSRKQNNPLLIHVDTKAGHGAGKPTAKVIEEVSDMFAFIARCLNIDWIP
|
|
|
|---|
| BDBM50134158 |
|---|
| n/a |
|---|
| Name | BDBM50134158 |
|---|
| Synonyms: | CHEMBL142133 | N-((R)-1-Methyl-2-oxo-2-pyrrolidin-1-yl-ethyl)-N'-((S)-1-methyl-2-oxo-2-pyrrolidin-1-yl-ethyl)-isophthalamide |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C22H30N4O4 |
|---|
| Mol. Mass. | 414.498 |
|---|
| SMILES | C[C@@H](NC(=O)c1cccc(c1)C(=O)N[C@@H](C)C(=O)N1CCCC1)C(=O)N1CCCC1 |
|---|
| Structure | 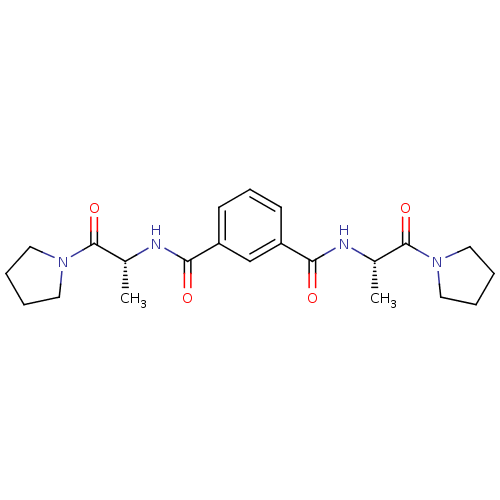
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Wallén, EA; Christiaans, JA; Jarho, EM; Forsberg, MM; Venäläinen, JI; Männistö, PT; Gynther, J New prolyl oligopeptidase inhibitors developed from dicarboxylic acid bis(l-prolyl-pyrrolidine) amides. J Med Chem46:4543-51 (2003) [PubMed] Article
Wallén, EA; Christiaans, JA; Jarho, EM; Forsberg, MM; Venäläinen, JI; Männistö, PT; Gynther, J New prolyl oligopeptidase inhibitors developed from dicarboxylic acid bis(l-prolyl-pyrrolidine) amides. J Med Chem46:4543-51 (2003) [PubMed] Article