

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Reaction Details | |||
|---|---|---|---|
 | Report a problem with these data | ||
| Target | Muscarinic acetylcholine receptor M3 | ||
| Ligand | BDBM50569296 | ||
| Substrate/Competitor | n/a | ||
| Meas. Tech. | ChEMBL_2108812 (CHEMBL4817487) | ||
| Ki | 0.158489±n/a nM | ||
| Citation |  Rancati, F; Linney, ID; Rizzi, A; Delcanale, M; Knight, CK; Schmidt, W; Pastore, F; Riccardi, B; Mileo, V; Carnini, C; Cesari, N; Blackaby, WP; Patacchini, R; Carzaniga, L Discovery of a novel class of inhaled dual pharmacology muscarinic antagonist and ? Bioorg Med Chem Lett41:0 (2021) [PubMed] Article Rancati, F; Linney, ID; Rizzi, A; Delcanale, M; Knight, CK; Schmidt, W; Pastore, F; Riccardi, B; Mileo, V; Carnini, C; Cesari, N; Blackaby, WP; Patacchini, R; Carzaniga, L Discovery of a novel class of inhaled dual pharmacology muscarinic antagonist and ? Bioorg Med Chem Lett41:0 (2021) [PubMed] Article | ||
| More Info.: | Get all data from this article, Assay Method | ||
| Muscarinic acetylcholine receptor M3 | |||
| Name: | Muscarinic acetylcholine receptor M3 | ||
| Synonyms: | ACM3_HUMAN | CHRM3 | Cholinergic, muscarinic M3 | Muscarinic Receptors M3 | Muscarinic receptor M3 | RecName: Full=Muscarinic acetylcholine receptor M3 | ||
| Type: | Enzyme | ||
| Mol. Mass.: | 66151.03 | ||
| Organism: | Homo sapiens (Human) | ||
| Description: | P20309 | ||
| Residue: | 590 | ||
| Sequence: |
| ||
| BDBM50569296 | |||
| n/a | |||
| Name | BDBM50569296 | ||
| Synonyms: | CHEMBL4852629 | ||
| Type | Small organic molecule | ||
| Emp. Form. | C40H50N4O6 | ||
| Mol. Mass. | 682.8482 | ||
| SMILES | O[C@@H](CNCCCCCCCCOc1cccc(c1)C(NC(=O)O[C@H]1CN2CCC1CC2)c1ccccc1)c1ccc(O)c2[nH]c(=O)ccc12 |r,wU:1.0,24.24,(3.65,-24.81,;4.98,-25.58,;6.32,-24.81,;7.65,-25.57,;8.98,-24.8,;10.32,-25.56,;11.65,-24.79,;12.99,-25.56,;14.32,-24.78,;15.65,-25.55,;16.99,-24.77,;18.32,-25.54,;19.65,-24.77,;20.99,-25.42,;20.98,-26.95,;22.32,-27.72,;23.65,-26.94,;23.64,-25.4,;22.31,-24.64,;24.98,-24.61,;26.31,-25.37,;27.64,-24.6,;27.63,-23.06,;28.98,-25.36,;30.31,-24.58,;30.3,-23.05,;31.63,-22.28,;32.97,-23.05,;32.97,-24.59,;31.65,-25.36,;32.34,-24,;30.85,-23.61,;24.97,-23.08,;26.3,-22.31,;26.29,-20.77,;24.95,-20,;23.62,-20.79,;23.63,-22.32,;4.99,-27.12,;3.66,-27.89,;3.66,-29.44,;4.99,-30.21,;5,-31.75,;6.32,-29.43,;7.65,-30.2,;9,-29.43,;10.33,-30.21,;9,-27.89,;7.66,-27.11,;6.32,-27.88,)| | ||
| Structure | 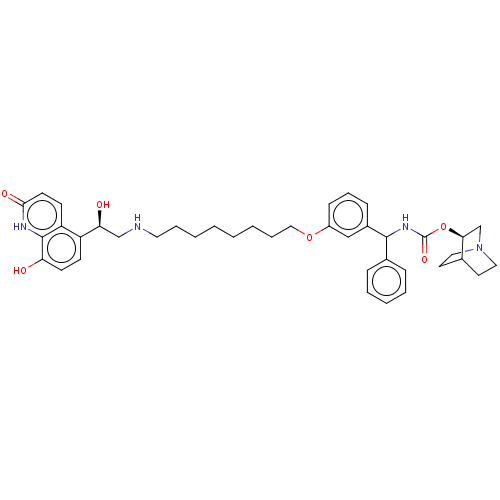
| ||