| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Cytochrome P450 3A4 |
|---|
| Ligand | BDBM50569645 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_2109894 (CHEMBL4818569) |
|---|
| IC50 | 1600±n/a nM |
|---|
| Citation |  Huang, SY; Wang, X; Shen, DY; Chen, F; Zhang, GY; Zhang, Z; Li, K; Jin, Z; Du, D; Tang, YZ Design, synthesis and biological evaluation of novel pleuromutilin derivatives as potent anti-MRSA agents targeting the 50S ribosome. Bioorg Med Chem38:0 (2021) [PubMed] Article Huang, SY; Wang, X; Shen, DY; Chen, F; Zhang, GY; Zhang, Z; Li, K; Jin, Z; Du, D; Tang, YZ Design, synthesis and biological evaluation of novel pleuromutilin derivatives as potent anti-MRSA agents targeting the 50S ribosome. Bioorg Med Chem38:0 (2021) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Cytochrome P450 3A4 |
|---|
| Name: | Cytochrome P450 3A4 |
|---|
| Synonyms: | Albendazole monooxygenase | Albendazole sulfoxidase | CP3A4_HUMAN | CYP3A3 | CYP3A4 | CYPIIIA3 | CYPIIIA4 | Cytochrome P450 3A3 | Cytochrome P450 3A4 (CYP3A4) | Cytochrome P450 HLp | Nifedipine oxidase | Quinine 3-monooxygenase | Taurochenodeoxycholate 6-alpha-hydroxylase |
|---|
| Type: | Enzyme |
|---|
| Mol. Mass.: | 57349.57 |
|---|
| Organism: | Homo sapiens (Human) |
|---|
| Description: | n/a |
|---|
| Residue: | 503 |
|---|
| Sequence: | MALIPDLAMETWLLLAVSLVLLYLYGTHSHGLFKKLGIPGPTPLPFLGNILSYHKGFCMF
DMECHKKYGKVWGFYDGQQPVLAITDPDMIKTVLVKECYSVFTNRRPFGPVGFMKSAISI
AEDEEWKRLRSLLSPTFTSGKLKEMVPIIAQYGDVLVRNLRREAETGKPVTLKDVFGAYS
MDVITSTSFGVNIDSLNNPQDPFVENTKKLLRFDFLDPFFLSITVFPFLIPILEVLNICV
FPREVTNFLRKSVKRMKESRLEDTQKHRVDFLQLMIDSQNSKETESHKALSDLELVAQSI
IFIFAGYETTSSVLSFIMYELATHPDVQQKLQEEIDAVLPNKAPPTYDTVLQMEYLDMVV
NETLRLFPIAMRLERVCKKDVEINGMFIPKGVVVMIPSYALHRDPKYWTEPEKFLPERFS
KKNKDNIDPYIYTPFGSGPRNCIGMRFALMNMKLALIRVLQNFSFKPCKETQIPLKLSLG
GLLQPEKPVVLKVESRDGTVSGA
|
|
|
|---|
| BDBM50569645 |
|---|
| n/a |
|---|
| Name | BDBM50569645 |
|---|
| Synonyms: | CHEBI:44137 | Denagard | SO 14055 | SQ 14055 | SQ-14055 | Tiamulin |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C28H47NO4S |
|---|
| Mol. Mass. | 493.742 |
|---|
| SMILES | [H][C@@]12C(=O)CC[C@]11CC[C@@H](C)[C@@]2(C)[C@@H](C[C@@](C)(C=C)[C@@H](O)[C@@H]1C)OC(=O)CSCCN(CC)CC |r| |
|---|
| Structure | 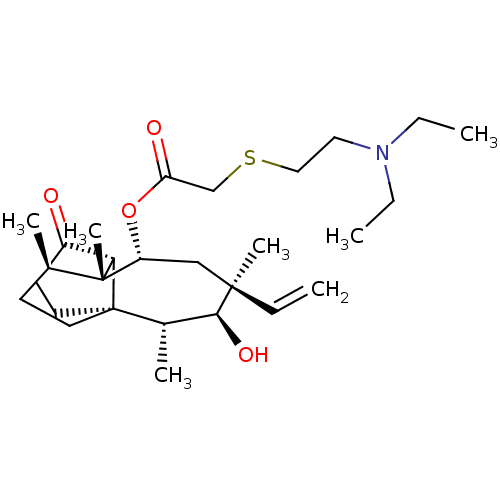
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Huang, SY; Wang, X; Shen, DY; Chen, F; Zhang, GY; Zhang, Z; Li, K; Jin, Z; Du, D; Tang, YZ Design, synthesis and biological evaluation of novel pleuromutilin derivatives as potent anti-MRSA agents targeting the 50S ribosome. Bioorg Med Chem38:0 (2021) [PubMed] Article
Huang, SY; Wang, X; Shen, DY; Chen, F; Zhang, GY; Zhang, Z; Li, K; Jin, Z; Du, D; Tang, YZ Design, synthesis and biological evaluation of novel pleuromutilin derivatives as potent anti-MRSA agents targeting the 50S ribosome. Bioorg Med Chem38:0 (2021) [PubMed] Article