| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Histone acetyltransferase KAT2A |
|---|
| Ligand | BDBM50573197 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_2119173 (CHEMBL4828239) |
|---|
| Kd | 44±n/a nM |
|---|
| Citation |  Zahid, H; Buchholz, CR; Singh, M; Ciccone, MF; Chan, A; Nithianantham, S; Shi, K; Aihara, H; Fischer, M; Schönbrunn, E; Dos Santos, CO; Landry, JW; Pomerantz, WCK New Design Rules for Developing Potent Cell-Active Inhibitors of the Nucleosome Remodeling Factor (NURF) via BPTF Bromodomain Inhibition. J Med Chem64:13902-13917 (2021) [PubMed] Article Zahid, H; Buchholz, CR; Singh, M; Ciccone, MF; Chan, A; Nithianantham, S; Shi, K; Aihara, H; Fischer, M; Schönbrunn, E; Dos Santos, CO; Landry, JW; Pomerantz, WCK New Design Rules for Developing Potent Cell-Active Inhibitors of the Nucleosome Remodeling Factor (NURF) via BPTF Bromodomain Inhibition. J Med Chem64:13902-13917 (2021) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Histone acetyltransferase KAT2A |
|---|
| Name: | Histone acetyltransferase KAT2A |
|---|
| Synonyms: | GCN5 | GCN5 | GCN5L2 | General control of amino acid synthesis protein 5-like 2 | HGCN5 | Histone acetyltransferase GCN5 | Histone acetyltransferase KAT2A | Histone acetyltransferase KAT2A/KAT2B | HsGCN5 | KAT2A | KAT2A_HUMAN | Lysine acetyltransferase 2A | STAF97 |
|---|
| Type: | PROTEIN |
|---|
| Mol. Mass.: | 93956.22 |
|---|
| Organism: | Homo sapiens (Human) |
|---|
| Description: | ChEMBL_100876 |
|---|
| Residue: | 837 |
|---|
| Sequence: | MAEPSQAPTPAPAAQPRPLQSPAPAPTPTPAPSPASAPIPTPTPAPAPAPAAAPAGSTGT
GGPGVGSGGAGSGGDPARPGLSQQQRASQRKAQVRGLPRAKKLEKLGVFSACKANETCKC
NGWKNPKPPTAPRMDLQQPAANLSELCRSCEHPLADHVSHLENVSEDEINRLLGMVVDVE
NLFMSVHKEEDTDTKQVYFYLFKLLRKCILQMTRPVVEGSLGSPPFEKPNIEQGVLNFVQ
YKFSHLAPRERQTMFELSKMFLLCLNYWKLETPAQFRQRSQAEDVATYKVNYTRWLCYCH
VPQSCDSLPRYETTHVFGRSLLRSIFTVTRRQLLEKFRVEKDKLVPEKRTLILTHFPKFL
SMLEEEIYGANSPIWESGFTMPPSEGTQLVPRPASVSAAVVPSTPIFSPSMGGGSNSSLS
LDSAGAEPMPGEKRTLPENLTLEDAKRLRVMGDIPMELVNEVMLTITDPAAMLGPETSLL
SANAARDETARLEERRGIIEFHVIGNSLTPKANRRVLLWLVGLQNVFSHQLPRMPKEYIA
RLVFDPKHKTLALIKDGRVIGGICFRMFPTQGFTEIVFCAVTSNEQVKGYGTHLMNHLKE
YHIKHNILYFLTYADEYAIGYFKKQGFSKDIKVPKSRYLGYIKDYEGATLMECELNPRIP
YTELSHIIKKQKEIIKKLIERKQAQIRKVYPGLSCFKEGVRQIPVESVPGIRETGWKPLG
KEKGKELKDPDQLYTTLKNLLAQIKSHPSAWPFMEPVKKSEAPDYYEVIRFPIDLKTMTE
RLRSRYYVTRKLFVADLQRVIANCREYNPPDSEYCRCASALEKFFYFKLKEGGLIDK
|
|
|
|---|
| BDBM50573197 |
|---|
| n/a |
|---|
| Name | BDBM50573197 |
|---|
| Synonyms: | CHEMBL4872300 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C13H15ClN4O |
|---|
| Mol. Mass. | 278.737 |
|---|
| SMILES | Cn1ncc(Nc2ccc(CCN)cc2)c(Cl)c1=O |
|---|
| Structure | 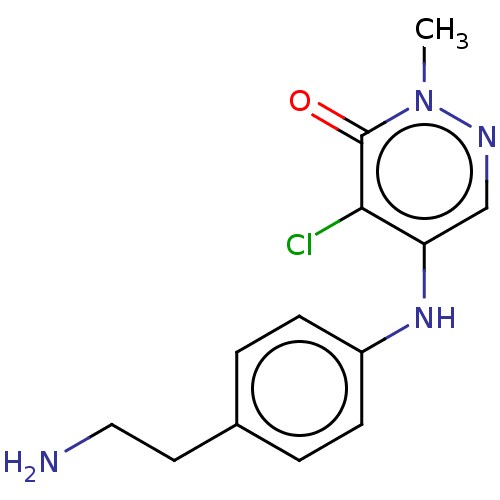
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Zahid, H; Buchholz, CR; Singh, M; Ciccone, MF; Chan, A; Nithianantham, S; Shi, K; Aihara, H; Fischer, M; Schönbrunn, E; Dos Santos, CO; Landry, JW; Pomerantz, WCK New Design Rules for Developing Potent Cell-Active Inhibitors of the Nucleosome Remodeling Factor (NURF) via BPTF Bromodomain Inhibition. J Med Chem64:13902-13917 (2021) [PubMed] Article
Zahid, H; Buchholz, CR; Singh, M; Ciccone, MF; Chan, A; Nithianantham, S; Shi, K; Aihara, H; Fischer, M; Schönbrunn, E; Dos Santos, CO; Landry, JW; Pomerantz, WCK New Design Rules for Developing Potent Cell-Active Inhibitors of the Nucleosome Remodeling Factor (NURF) via BPTF Bromodomain Inhibition. J Med Chem64:13902-13917 (2021) [PubMed] Article