| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Amyloid-beta precursor protein |
|---|
| Ligand | BDBM16034 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_2129519 (CHEMBL4838948) |
|---|
| IC50 | >100000±n/a nM |
|---|
| Citation |  Masuda, Y; Fujihara, K; Hayashi, S; Sasaki, H; Kino, Y; Kamauchi, H; Noji, M; Satoh, JI; Takanami, T; Kinoshita, K; Koyama, K Inhibition of BACE1 and Amyloid-? Aggregation by Meroterpenoids from the Mushroom J Nat Prod84:1748-1754 (2021) [PubMed] Article Masuda, Y; Fujihara, K; Hayashi, S; Sasaki, H; Kino, Y; Kamauchi, H; Noji, M; Satoh, JI; Takanami, T; Kinoshita, K; Koyama, K Inhibition of BACE1 and Amyloid-? Aggregation by Meroterpenoids from the Mushroom J Nat Prod84:1748-1754 (2021) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Amyloid-beta precursor protein |
|---|
| Name: | Amyloid-beta precursor protein |
|---|
| Synonyms: | A4 | A4_HUMAN | ABPP | AD1 | AICD-50 | AICD-57 | AICD-59 | AID(50) | AID(57) | AID(59) | APP | APPI | Alzheimer disease amyloid protein | Amyloid beta A4 protein | Amyloid beta Protein | Amyloid beta protein (sAPPbeta) | Amyloid beta protein Abeta(1-42) | Amyloid intracellular domain 50 | Amyloid intracellular domain 57 | Amyloid intracellular domain 59 | Amyloid protein (Abeta42b) | Amyloid β-protein (Aβ42) | Beta amyloid A4 protein | Beta-APP40 | Beta-APP42 | Beta-amyloid protein 40 | Beta-amyloid protein 42 | C31 | C83 | C99 | CVAP | Cerebral vascular amyloid peptide | Gamma Secretase | Gamma-CTF(50) | Gamma-CTF(57) | Gamma-CTF(59) | Gamma-secretase | Gamma-secretase C-terminal fragment 50 | Gamma-secretase C-terminal fragment 57 | Gamma-secretase C-terminal fragment 59 | P3(40) | P3(42) | PN-II | PreA4 | Protease nexin-II | S-APP-alpha | S-APP-beta | Soluble APP-alpha | Soluble APP-beta | beta-Amyloid Precursor Protein (APP) |
|---|
| Type: | Single-pass type I membrane protein |
|---|
| Mol. Mass.: | 86890.41 |
|---|
| Organism: | Homo sapiens (Human) |
|---|
| Description: | P05067 |
|---|
| Residue: | 770 |
|---|
| Sequence: | MLPGLALLLLAAWTARALEVPTDGNAGLLAEPQIAMFCGRLNMHMNVQNGKWDSDPSGTK
TCIDTKEGILQYCQEVYPELQITNVVEANQPVTIQNWCKRGRKQCKTHPHFVIPYRCLVG
EFVSDALLVPDKCKFLHQERMDVCETHLHWHTVAKETCSEKSTNLHDYGMLLPCGIDKFR
GVEFVCCPLAEESDNVDSADAEEDDSDVWWGGADTDYADGSEDKVVEVAEEEEVAEVEEE
EADDDEDDEDGDEVEEEAEEPYEEATERTTSIATTTTTTTESVEEVVREVCSEQAETGPC
RAMISRWYFDVTEGKCAPFFYGGCGGNRNNFDTEEYCMAVCGSAMSQSLLKTTQEPLARD
PVKLPTTAASTPDAVDKYLETPGDENEHAHFQKAKERLEAKHRERMSQVMREWEEAERQA
KNLPKADKKAVIQHFQEKVESLEQEAANERQQLVETHMARVEAMLNDRRRLALENYITAL
QAVPPRPRHVFNMLKKYVRAEQKDRQHTLKHFEHVRMVDPKKAAQIRSQVMTHLRVIYER
MNQSLSLLYNVPAVAEEIQDEVDELLQKEQNYSDDVLANMISEPRISYGNDALMPSLTET
KTTVELLPVNGEFSLDDLQPWHSFGADSVPANTENEVEPVDARPAADRGLTTRPGSGLTN
IKTEEISEVKMDAEFRHDSGYEVHHQKLVFFAEDVGSNKGAIIGLMVGGVVIATVIVITL
VMLKKKQYTSIHHGVVEVDAAVTPEERHLSKMQQNGYENPTYKFFEQMQN
|
|
|
|---|
| BDBM16034 |
|---|
| n/a |
|---|
| Name | BDBM16034 |
|---|
| Synonyms: | 1-N-[(2S,3R)-4-(cyclopropylamino)-3-hydroxy-1-phenylbutan-2-yl]-5-(N-methylmethanesulfonamido)-3-N-[(1R)-1-phenylethyl]benzene-1,3-dicarboxamide | 5-Substituted isophthalamide, 1 | CHEMBL378225 | Isophthalamide Derivative 24 | L-000384950 | N-[(1S,2R)-1-benzyl-3-(cyclopropylamino)-2-hydroxypropyl]-5-[methyl(methylsulfonyl)amino]-N-[(1R)-1-phenylethyl]benzene-1,3-dicarboxamide | hydroxyethylamine (HEA) derived inhibitor 1 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C31H38N4O5S |
|---|
| Mol. Mass. | 578.722 |
|---|
| SMILES | C[C@@H](NC(=O)c1cc(cc(c1)C(=O)N[C@@H](Cc1ccccc1)[C@H](O)CNC1CC1)N(C)S(C)(=O)=O)c1ccccc1 |r| |
|---|
| Structure | 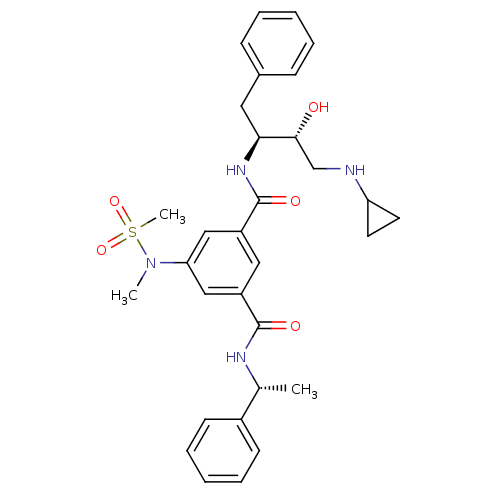
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Masuda, Y; Fujihara, K; Hayashi, S; Sasaki, H; Kino, Y; Kamauchi, H; Noji, M; Satoh, JI; Takanami, T; Kinoshita, K; Koyama, K Inhibition of BACE1 and Amyloid-? Aggregation by Meroterpenoids from the Mushroom J Nat Prod84:1748-1754 (2021) [PubMed] Article
Masuda, Y; Fujihara, K; Hayashi, S; Sasaki, H; Kino, Y; Kamauchi, H; Noji, M; Satoh, JI; Takanami, T; Kinoshita, K; Koyama, K Inhibition of BACE1 and Amyloid-? Aggregation by Meroterpenoids from the Mushroom J Nat Prod84:1748-1754 (2021) [PubMed] Article