| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Endothelin-1 receptor |
|---|
| Ligand | BDBM50112678 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEBML_63215 |
|---|
| IC50 | 0.190000±n/a nM |
|---|
| Citation |  Funk, OF; Kettmann, V; Drimal, J; Langer, T Chemical function based pharmacophore generation of endothelin-A selective receptor antagonists. J Med Chem47:2750-60 (2004) [PubMed] Article Funk, OF; Kettmann, V; Drimal, J; Langer, T Chemical function based pharmacophore generation of endothelin-A selective receptor antagonists. J Med Chem47:2750-60 (2004) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Endothelin-1 receptor |
|---|
| Name: | Endothelin-1 receptor |
|---|
| Synonyms: | EDNRA_RAT | ENDOTHELIN A | Ednra | Endothelin receptor | Endothelin-1 receptor |
|---|
| Type: | Enzyme Catalytic Domain |
|---|
| Mol. Mass.: | 48256.91 |
|---|
| Organism: | RAT |
|---|
| Description: | ENDOTHELIN A EDNRA RAT::P26684 |
|---|
| Residue: | 426 |
|---|
| Sequence: | MGVLCFLASFWLALVGGAIADNAERYSANLSSHVEDFTPFPGTEFNFLGTTLQPPNLALP
SNGSMHGYCPQQTKITTAFKYINTVISCTIFIVGMVGNATLLRIIYQNKCMRNGPNALIA
SLALGDLIYVVIDLPINVFKLLAGRWPFDHNDFGVFLCKLFPFLQKSSVGITVLNLCALS
VDRYRAVASWSRVQGIGIPLITAIEIVSIWILSFILAIPEAIGFVMVPFEYKGEQHRTCM
LNATTKFMEFYQDVKDWWLFGFYFCMPLVCTAIFYTLMTCEMLNRRNGSLRIALSEHLKQ
RREVAKTVFCLVVIFALCWFPLHLSRILKKTVYDEMDKNRCELLSFLLLMDYIGINLATM
NSCINPIALYFVSKKFKNCFQSCLCCCCHQSKSLMTSVPMNGTSIQWKNQEQNHNTERSS
HKDSMN
|
|
|
|---|
| BDBM50112678 |
|---|
| n/a |
|---|
| Name | BDBM50112678 |
|---|
| Synonyms: | (R)-2-Benzo[1,3]dioxol-5-yl-6-isopropoxy-4-(4-methoxy-phenyl)-2H-chromene-3-carboxylic acid | 2-Benzo[1,3]dioxol-5-yl-6-isopropoxy-4-(4-methoxy-phenyl)-2H-chromene-3-carboxylic acid | CHEMBL61211 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C27H24O7 |
|---|
| Mol. Mass. | 460.4753 |
|---|
| SMILES | COc1ccc(cc1)C1=C([C@H](Oc2ccc(OC(C)C)cc12)c1ccc2OCOc2c1)C(O)=O |t:9| |
|---|
| Structure | 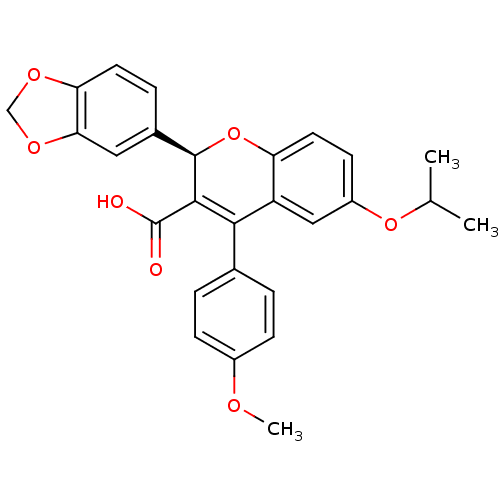
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Funk, OF; Kettmann, V; Drimal, J; Langer, T Chemical function based pharmacophore generation of endothelin-A selective receptor antagonists. J Med Chem47:2750-60 (2004) [PubMed] Article
Funk, OF; Kettmann, V; Drimal, J; Langer, T Chemical function based pharmacophore generation of endothelin-A selective receptor antagonists. J Med Chem47:2750-60 (2004) [PubMed] Article