

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Reaction Details | |||
|---|---|---|---|
 | Report a problem with these data | ||
| Target | Cytochrome P450 3A4 | ||
| Ligand | BDBM50585943 | ||
| Substrate/Competitor | n/a | ||
| Meas. Tech. | ChEMBL_2164346 (CHEMBL5049207) | ||
| IC50 | 1200±n/a nM | ||
| Citation |  Poddutoori, R; Aardalen, K; Aithal, K; Barahagar, SS; Belliappa, C; Bock, M; Chelur, S; Gerken, A; Gopinath, S; Gruenenfelder, B; Kiffe, M; Krishnaswami, M; Langowski, J; Madapa, S; Narayanan, K; Pandit, C; Panigrahi, SK; Perrone, M; Potakamuri, RK; Ramachandra, M; Ramanathan, A; Ramos, R; Sager, E; Samajdar, S; Subramanya, HS; Thimmasandra, DS; Venetsanakos, E; M÷bitz, H Discovery of MAP855, an Efficacious and Selective MEK1/2 Inhibitor with an ATP-Competitive Mode of Action. J Med Chem65:4350-4366 (2022) [PubMed] Article Poddutoori, R; Aardalen, K; Aithal, K; Barahagar, SS; Belliappa, C; Bock, M; Chelur, S; Gerken, A; Gopinath, S; Gruenenfelder, B; Kiffe, M; Krishnaswami, M; Langowski, J; Madapa, S; Narayanan, K; Pandit, C; Panigrahi, SK; Perrone, M; Potakamuri, RK; Ramachandra, M; Ramanathan, A; Ramos, R; Sager, E; Samajdar, S; Subramanya, HS; Thimmasandra, DS; Venetsanakos, E; M÷bitz, H Discovery of MAP855, an Efficacious and Selective MEK1/2 Inhibitor with an ATP-Competitive Mode of Action. J Med Chem65:4350-4366 (2022) [PubMed] Article | ||
| More Info.: | Get all data from this article, Assay Method | ||
| Cytochrome P450 3A4 | |||
| Name: | Cytochrome P450 3A4 | ||
| Synonyms: | Albendazole monooxygenase | Albendazole sulfoxidase | CP3A4_HUMAN | CYP3A3 | CYP3A4 | CYPIIIA3 | CYPIIIA4 | Cytochrome P450 3A3 | Cytochrome P450 3A4 (CYP3A4) | Cytochrome P450 HLp | Nifedipine oxidase | Quinine 3-monooxygenase | Taurochenodeoxycholate 6-alpha-hydroxylase | ||
| Type: | Enzyme | ||
| Mol. Mass.: | 57349.57 | ||
| Organism: | Homo sapiens (Human) | ||
| Description: | n/a | ||
| Residue: | 503 | ||
| Sequence: |
| ||
| BDBM50585943 | |||
| n/a | |||
| Name | BDBM50585943 | ||
| Synonyms: | CHEMBL5076921 | ||
| Type | Small organic molecule | ||
| Emp. Form. | C29H25ClFN7O | ||
| Mol. Mass. | 542.007 | ||
| SMILES | Cc1nc2cnc3cc(F)c(cc3c2n1[C@H]1CC[C@@](N)(CC#N)CC1)-c1ccc(Oc2ncccn2)cc1Cl |r,wU:15.17,wD:18.21,(-7.97,-1.41,;-7.2,-.08,;-7.83,1.33,;-6.69,2.36,;-6.69,3.89,;-5.36,4.67,;-4.02,3.9,;-2.69,4.66,;-1.36,3.9,;-.02,4.67,;-1.36,2.36,;-2.69,1.59,;-4.02,2.36,;-5.35,1.59,;-5.67,.08,;-4.58,-1.01,;-3.09,-.61,;-2.01,-1.7,;-2.4,-3.19,;-2.8,-4.67,;-1.32,-4.27,;.17,-3.88,;1.66,-3.48,;-3.89,-3.58,;-4.98,-2.49,;-.02,1.59,;-.02,.04,;1.31,-.72,;2.64,.05,;3.97,-.72,;5.31,.05,;5.31,1.59,;6.64,2.35,;7.97,1.58,;7.97,.05,;6.64,-.73,;2.64,1.59,;1.31,2.36,;1.31,3.9,)| | ||
| Structure | 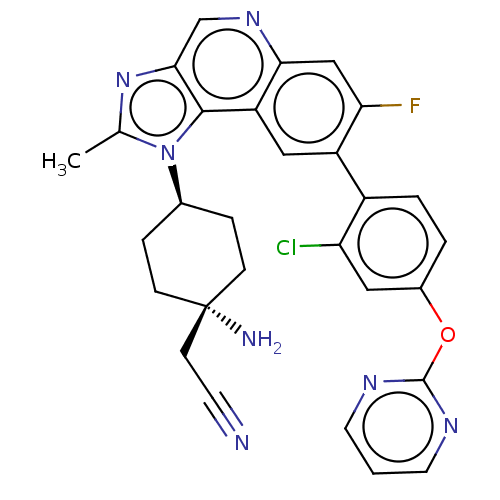
| ||