| Reaction Details |
|---|
 | Report a problem with these data |
| Target | 5-hydroxytryptamine receptor 2C |
|---|
| Ligand | BDBM50158032 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_303338 (CHEMBL840154) |
|---|
| Ki | 383±n/a nM |
|---|
| Citation |  Cole, DC; Ellingboe, JW; Lennox, WJ; Mazandarani, H; Smith, DL; Stock, JR; Zhang, G; Zhou, P; Schechter, LE N1-arylsulfonyl-3-(1,2,3,6-tetrahydropyridin-4-yl)-1H-indole derivatives are potent and selective 5-HT6 receptor antagonists. Bioorg Med Chem Lett15:379-83 (2004) [PubMed] Article Cole, DC; Ellingboe, JW; Lennox, WJ; Mazandarani, H; Smith, DL; Stock, JR; Zhang, G; Zhou, P; Schechter, LE N1-arylsulfonyl-3-(1,2,3,6-tetrahydropyridin-4-yl)-1H-indole derivatives are potent and selective 5-HT6 receptor antagonists. Bioorg Med Chem Lett15:379-83 (2004) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| 5-hydroxytryptamine receptor 2C |
|---|
| Name: | 5-hydroxytryptamine receptor 2C |
|---|
| Synonyms: | 5-HT-1C | 5-HT-2C | 5-HT1C | 5-HT2C | 5-HT2C-INI | 5-HT2c VGI | 5-HTR2C | 5-hydroxytryptamine receptor 1C | 5-hydroxytryptamine receptor 2C (5-HT-2C) | 5-hydroxytryptamine receptor 2C (5HT-2C) | 5HT-1C | 5HT2C_HUMAN | HTR1C | HTR2C | Serotonin (5-HT3) receptor | Serotonin 2c (5-HT2c) receptor | Serotonin Receptor 2C |
|---|
| Type: | G Protein-Coupled Receptor (GPCR) |
|---|
| Mol. Mass.: | 51836.79 |
|---|
| Organism: | Homo sapiens (Human) |
|---|
| Description: | P28335 |
|---|
| Residue: | 458 |
|---|
| Sequence: | MVNLRNAVHSFLVHLIGLLVWQSDISVSPVAAIVTDIFNTSDGGRFKFPDGVQNWPALSI
VIIIIMTIGGNILVIMAVSMEKKLHNATNYFLMSLAIADMLVGLLVMPLSLLAILYDYVW
PLPRYLCPVWISLDVLFSTASIMHLCAISLDRYVAIRNPIEHSRFNSRTKAIMKIAIVWA
ISIGVSVPIPVIGLRDEEKVFVNNTTCVLNDPNFVLIGSFVAFFIPLTIMVITYCLTIYV
LRRQALMLLHGHTEEPPGLSLDFLKCCKRNTAEEENSANPNQDQNARRRKKKERRPRGTM
QAINNERKASKVLGIVFFVFLIMWCPFFITNILSVLCEKSCNQKLMEKLLNVFVWIGYVC
SGINPLVYTLFNKIYRRAFSNYLRCNYKVEKKPPVRQIPRVAATALSGRELNVNIYRHTN
EPVIEKASDNEPGIEMQVENLELPVNPSSVVSERISSV
|
|
|
|---|
| BDBM50158032 |
|---|
| n/a |
|---|
| Name | BDBM50158032 |
|---|
| Synonyms: | 1-(2-Chloro-benzenesulfonyl)-3-(1,2,3,6-tetrahydro-pyridin-4-yl)-1H-indole | CHEMBL362487 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C19H17ClN2O2S |
|---|
| Mol. Mass. | 372.868 |
|---|
| SMILES | Clc1ccccc1S(=O)(=O)n1cc(C2=CCNCC2)c2ccccc12 |t:14| |
|---|
| Structure | 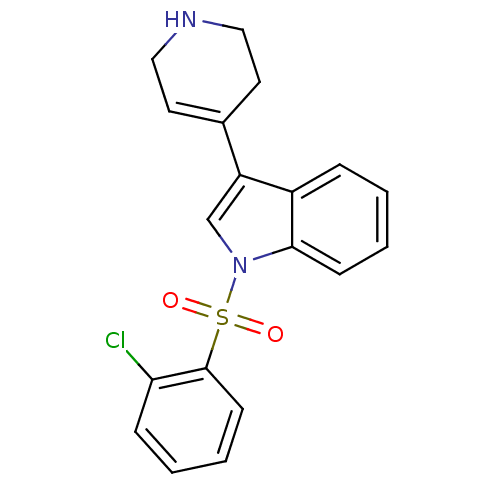
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Cole, DC; Ellingboe, JW; Lennox, WJ; Mazandarani, H; Smith, DL; Stock, JR; Zhang, G; Zhou, P; Schechter, LE N1-arylsulfonyl-3-(1,2,3,6-tetrahydropyridin-4-yl)-1H-indole derivatives are potent and selective 5-HT6 receptor antagonists. Bioorg Med Chem Lett15:379-83 (2004) [PubMed] Article
Cole, DC; Ellingboe, JW; Lennox, WJ; Mazandarani, H; Smith, DL; Stock, JR; Zhang, G; Zhou, P; Schechter, LE N1-arylsulfonyl-3-(1,2,3,6-tetrahydropyridin-4-yl)-1H-indole derivatives are potent and selective 5-HT6 receptor antagonists. Bioorg Med Chem Lett15:379-83 (2004) [PubMed] Article