| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Ephrin type-A receptor 2 |
|---|
| Ligand | BDBM50588615 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_2187820 (CHEMBL5099902) |
|---|
| IC50 | 27±n/a nM |
|---|
| Citation |  Baggio, C; Udompholkul, P; Gambini, L; Pellecchia, M Targefrin: A Potent Agent Targeting the Ligand Binding Domain of EphA2. J Med Chem65:15443-15456 (2022) [PubMed] Article Baggio, C; Udompholkul, P; Gambini, L; Pellecchia, M Targefrin: A Potent Agent Targeting the Ligand Binding Domain of EphA2. J Med Chem65:15443-15456 (2022) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Ephrin type-A receptor 2 |
|---|
| Name: | Ephrin type-A receptor 2 |
|---|
| Synonyms: | ECK | EPHA2 | EPHA2_HUMAN | Ephrin receptor | Epithelial cell kinase | Tyrosine-protein kinase receptor ECK |
|---|
| Type: | PROTEIN |
|---|
| Mol. Mass.: | 108260.70 |
|---|
| Organism: | Homo sapiens (Human) |
|---|
| Description: | ChEMBL_1505248 |
|---|
| Residue: | 976 |
|---|
| Sequence: | MELQAARACFALLWGCALAAAAAAQGKEVVLLDFAAAGGELGWLTHPYGKGWDLMQNIMN
DMPIYMYSVCNVMSGDQDNWLRTNWVYRGEAERIFIELKFTVRDCNSFPGGASSCKETFN
LYYAESDLDYGTNFQKRLFTKIDTIAPDEITVSSDFEARHVKLNVEERSVGPLTRKGFYL
AFQDIGACVALLSVRVYYKKCPELLQGLAHFPETIAGSDAPSLATVAGTCVDHAVVPPGG
EEPRMHCAVDGEWLVPIGQCLCQAGYEKVEDACQACSPGFFKFEASESPCLECPEHTLPS
PEGATSCECEEGFFRAPQDPASMPCTRPPSAPHYLTAVGMGAKVELRWTPPQDSGGREDI
VYSVTCEQCWPESGECGPCEASVRYSEPPHGLTRTSVTVSDLEPHMNYTFTVEARNGVSG
LVTSRSFRTASVSINQTEPPKVRLEGRSTTSLSVSWSIPPPQQSRVWKYEVTYRKKGDSN
SYNVRRTEGFSVTLDDLAPDTTYLVQVQALTQEGQGAGSKVHEFQTLSPEGSGNLAVIGG
VAVGVVLLLVLAGVGFFIHRRRKNQRARQSPEDVYFSKSEQLKPLKTYVDPHTYEDPNQA
VLKFTTEIHPSCVTRQKVIGAGEFGEVYKGMLKTSSGKKEVPVAIKTLKAGYTEKQRVDF
LGEAGIMGQFSHHNIIRLEGVISKYKPMMIITEYMENGALDKFLREKDGEFSVLQLVGML
RGIAAGMKYLANMNYVHRDLAARNILVNSNLVCKVSDFGLSRVLEDDPEATYTTSGGKIP
IRWTAPEAISYRKFTSASDVWSFGIVMWEVMTYGERPYWELSNHEVMKAINDGFRLPTPM
DCPSAIYQLMMQCWQQERARRPKFADIVSILDKLIRAPDSLKTLADFDPRVSIRLPSTSG
SEGVPFRTVSEWLESIKMQQYTEHFMAAGYTAIEKVVQMTNDDIKRIGVRLPGHQKRIAY
SLLGLKDQVNTVGIPI
|
|
|
|---|
| BDBM50588615 |
|---|
| n/a |
|---|
| Name | BDBM50588615 |
|---|
| Synonyms: | CHEMBL5203641 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C247H328F6N48O51 |
|---|
| Mol. Mass. | 4899.5286 |
|---|
| SMILES | [H][C@@]12C[C@H](O)[C@@]3(C)C(=O)[C@H](OC(C)=O)C4=C(C)[C@H](C[C@@](O)([C@@H](OC(=O)c5ccccc5)[C@]3([H])[C@@]1(CO2)OC(C)=O)C4(C)C)OC(=O)[C@H](OC(=O)CCCCCn1cc(CCCC(=O)NCCCC[C@H](NC(=O)CNC(=O)[C@H](CCCCNC(=O)CNC(=O)[C@@H]2CCCN2C(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@@H]2CCCN2C(=O)[C@@H](NC(=O)[C@H](C)NC(=O)[C@H](CC(O)=O)NC(=O)[C@@H]2CCCN2C(=O)[C@H](Cc2ccc(cc2)-c2ccccc2C(F)(F)F)NC(=O)[C@H](C)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](Cc2ccc(N)cc2)NC(=O)CN2CCNCC2)C2CCCCC2)NC(=O)CNC(=O)[C@@H]2CCCN2C(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@@H]2CCCN2C(=O)[C@@H](NC(=O)[C@H](C)NC(=O)[C@H](CC(O)=O)NC(=O)[C@@H]2CCCN2C(=O)[C@H](Cc2ccc(cc2)-c2ccccc2C(F)(F)F)NC(=O)[C@H](C)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](Cc2ccc(N)cc2)NC(=O)CN2CCNCC2)C2CCCCC2)C(N)=O)nn1)[C@@H](NC(=O)c1ccccc1)c1ccccc1 |r,c:14| |
|---|
| Structure | 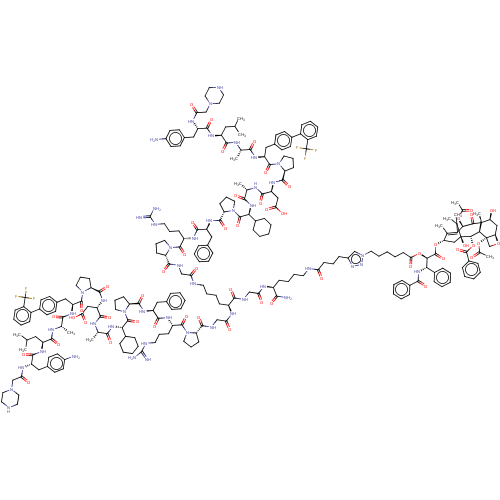
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Baggio, C; Udompholkul, P; Gambini, L; Pellecchia, M Targefrin: A Potent Agent Targeting the Ligand Binding Domain of EphA2. J Med Chem65:15443-15456 (2022) [PubMed] Article
Baggio, C; Udompholkul, P; Gambini, L; Pellecchia, M Targefrin: A Potent Agent Targeting the Ligand Binding Domain of EphA2. J Med Chem65:15443-15456 (2022) [PubMed] Article