| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Metabotropic glutamate receptor 2 |
|---|
| Ligand | BDBM50162571 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_304142 (CHEMBL840258) |
|---|
| EC50 | 442±n/a nM |
|---|
| Citation |  Pinkerton, AB; Cube, RV; Hutchinson, JH; James, JK; Gardner, MF; Rowe, BA; Schaffhauser, H; Rodriguez, DE; Campbell, UC; Daggett, LP; Vernier, JM Allosteric potentiators of the metabotropic glutamate receptor 2 (mGlu2). Part 3: Identification and biological activity of indanone containing mGlu2 receptor potentiators. Bioorg Med Chem Lett15:1565-71 (2005) [PubMed] Article Pinkerton, AB; Cube, RV; Hutchinson, JH; James, JK; Gardner, MF; Rowe, BA; Schaffhauser, H; Rodriguez, DE; Campbell, UC; Daggett, LP; Vernier, JM Allosteric potentiators of the metabotropic glutamate receptor 2 (mGlu2). Part 3: Identification and biological activity of indanone containing mGlu2 receptor potentiators. Bioorg Med Chem Lett15:1565-71 (2005) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Metabotropic glutamate receptor 2 |
|---|
| Name: | Metabotropic glutamate receptor 2 |
|---|
| Synonyms: | GPRC1B | GRM2 | GRM2_HUMAN | MGLUR2 | Metabotropic glutamate receptor | glutamate receptor, metabotropic 2 precursor | mGlu2 | metabotropic glutamate 2 |
|---|
| Type: | Enzyme |
|---|
| Mol. Mass.: | 95584.88 |
|---|
| Organism: | Homo sapiens (Human) |
|---|
| Description: | Q14416 |
|---|
| Residue: | 872 |
|---|
| Sequence: | MGSLLALLALLLLWGAVAEGPAKKVLTLEGDLVLGGLFPVHQKGGPAEDCGPVNEHRGIQ
RLEAMLFALDRINRDPHLLPGVRLGAHILDSCSKDTHALEQALDFVRASLSRGADGSRHI
CPDGSYATHGDAPTAITGVIGGSYSDVSIQVANLLRLFQIPQISYASTSAKLSDKSRYDY
FARTVPPDFFQAKAMAEILRFFNWTYVSTVASEGDYGETGIEAFELEARARNICVATSEK
VGRAMSRAAFEGVVRALLQKPSARVAVLFTRSEDARELLAASQRLNASFTWVASDGWGAL
ESVVAGSEGAAEGAITIELASYPISDFASYFQSLDPWNNSRNPWFREFWEQRFRCSFRQR
DCAAHSLRAVPFEQESKIMFVVNAVYAMAHALHNMHRALCPNTTRLCDAMRPVNGRRLYK
DFVLNVKFDAPFRPADTHNEVRFDRFGDGIGRYNIFTYLRAGSGRYRYQKVGYWAEGLTL
DTSLIPWASPSAGPLPASRCSEPCLQNEVKSVQPGEVCCWLCIPCQPYEYRLDEFTCADC
GLGYWPNASLTGCFELPQEYIRWGDAWAVGPVTIACLGALATLFVLGVFVRHNATPVVKA
SGRELCYILLGGVFLCYCMTFIFIAKPSTAVCTLRRLGLGTAFSVCYSALLTKTNRIARI
FGGAREGAQRPRFISPASQVAICLALISGQLLIVVAWLVVEAPGTGKETAPERREVVTLR
CNHRDASMLGSLAYNVLLIALCTLYAFKTRKCPENFNEAKFIGFTMYTTCIIWLAFLPIF
YVTSSDYRVQTTTMCVSVSLSGSVVLGCLFAPKLHIILFQPQKNVVSHRAPTSRFGSAAA
RASSSLGQGSGSQFVPTVCNGREVVDSTTSSL
|
|
|
|---|
| BDBM50162571 |
|---|
| n/a |
|---|
| Name | BDBM50162571 |
|---|
| Synonyms: | CHEMBL180455 | N-Acetyl-4-(6,7-dichloro-2-cyclopentyl-2-methyl-1-oxo-indan-5-yloxymethyl)-benzamide |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C25H25Cl2NO4 |
|---|
| Mol. Mass. | 474.376 |
|---|
| SMILES | CC(=O)NC(=O)c1ccc(COc2cc3CC(C)(C4CCCC4)C(=O)c3c(Cl)c2Cl)cc1 |
|---|
| Structure | 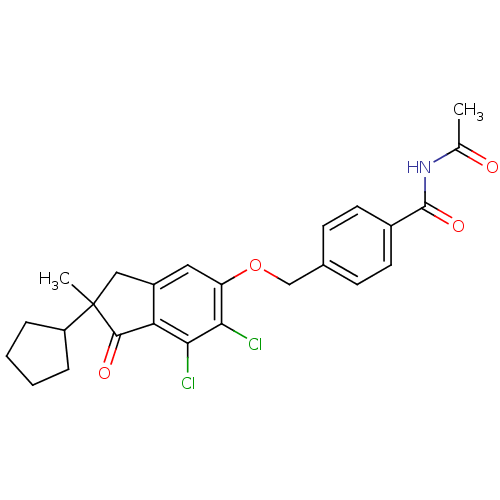
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Pinkerton, AB; Cube, RV; Hutchinson, JH; James, JK; Gardner, MF; Rowe, BA; Schaffhauser, H; Rodriguez, DE; Campbell, UC; Daggett, LP; Vernier, JM Allosteric potentiators of the metabotropic glutamate receptor 2 (mGlu2). Part 3: Identification and biological activity of indanone containing mGlu2 receptor potentiators. Bioorg Med Chem Lett15:1565-71 (2005) [PubMed] Article
Pinkerton, AB; Cube, RV; Hutchinson, JH; James, JK; Gardner, MF; Rowe, BA; Schaffhauser, H; Rodriguez, DE; Campbell, UC; Daggett, LP; Vernier, JM Allosteric potentiators of the metabotropic glutamate receptor 2 (mGlu2). Part 3: Identification and biological activity of indanone containing mGlu2 receptor potentiators. Bioorg Med Chem Lett15:1565-71 (2005) [PubMed] Article