| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Alpha-2A adrenergic receptor [16-465] |
|---|
| Ligand | BDBM50163104 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_302984 (CHEMBL828833) |
|---|
| Ki | 1000000±n/a nM |
|---|
| Citation |  Grunewald, GL; Romero, FA; Criscione, KR Nanomolar inhibitors of CNS epinephrine biosynthesis: (R)-(+)-3-fluoromethyl-7-(N-substituted aminosulfonyl)-1,2,3,4-tetrahydroisoquinolines as potent and highly selective inhibitors of phenylethanolamine N-methyltransferase1. J Med Chem48:1806-12 (2005) [PubMed] Article Grunewald, GL; Romero, FA; Criscione, KR Nanomolar inhibitors of CNS epinephrine biosynthesis: (R)-(+)-3-fluoromethyl-7-(N-substituted aminosulfonyl)-1,2,3,4-tetrahydroisoquinolines as potent and highly selective inhibitors of phenylethanolamine N-methyltransferase1. J Med Chem48:1806-12 (2005) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Alpha-2A adrenergic receptor [16-465] |
|---|
| Name: | Alpha-2A adrenergic receptor [16-465] |
|---|
| Synonyms: | ADA2A_RAT | Adra2a | Adrenaline 2 | Alpha-2A adrenergic receptor | Alpha-2A adrenoceptor | Alpha-2A adrenoreceptor | Alpha-2AAR | Alpha-2D adrenergic receptor | Alpha2 Adrenoreceptor | CA2-47 | adrenergic Alpha2A |
|---|
| Type: | G-protein coupled receptor |
|---|
| Mol. Mass.: | 48961.69 |
|---|
| Organism: | Rattus norvegicus (rat) |
|---|
| Description: | P22909[16-465] |
|---|
| Residue: | 450 |
|---|
| Sequence: | MGSLQPDAGNSSWNGTEAPGGGTRATPYSLQVTLTLVCLAGLLMLFTVFGNVLVIIAVFT
SRALKAPQNLFLVSLASADILVATLVIPFSLANEVMGYWYFGKVWCEIYLALDVLFCTSS
IVHLCAISLDRYWSITQAIEYNLKRTPRRIKAIIVTVWVISAVISFPPLISIEKKGAGGG
QQPAEPSCKINDQKWYVISSSIGSFFAPCLIMILVYVRIYQIAKRRTRVPPSRRGPDACS
APPGGADRRPNGLGPERGAGTAGAEAEPLPTQLNGAPGEPAPTRPRDGDALDLEESSSSE
HAERPQGPGKPERGPRAKGKTKASQVKPGDSLPRRGPGAAGPGASGSGQGEERAGGAKAS
RWRGRQNREKRFTFVLAVVIGVFVVCWFPFFFTYTLIAVGCPVPYQLFNFFFWFGYCNSS
LNPVIYTIFNHDFRRAFKKILCRGDRKRIV
|
|
|
|---|
| BDBM50163104 |
|---|
| n/a |
|---|
| Name | BDBM50163104 |
|---|
| Synonyms: | (R)-3-Fluoromethyl-1,2,3,4-tetrahydro-isoquinoline-7-sulfonic acid (2,2,2-trifluoro-ethyl)-amide | CHEMBL425522 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C12H14F4N2O2S |
|---|
| Mol. Mass. | 326.31 |
|---|
| SMILES | FC[C@H]1Cc2ccc(cc2CN1)S(=O)(=O)NCC(F)(F)F |
|---|
| Structure | 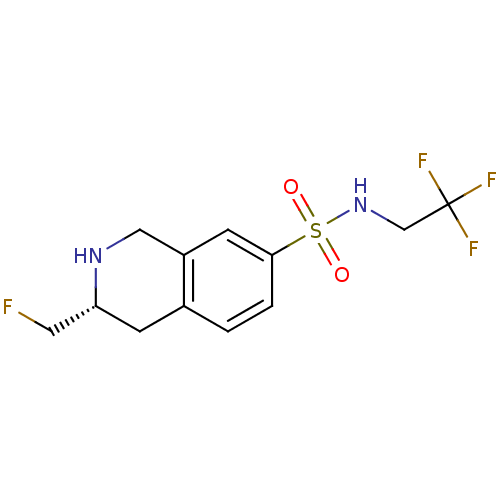
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Grunewald, GL; Romero, FA; Criscione, KR Nanomolar inhibitors of CNS epinephrine biosynthesis: (R)-(+)-3-fluoromethyl-7-(N-substituted aminosulfonyl)-1,2,3,4-tetrahydroisoquinolines as potent and highly selective inhibitors of phenylethanolamine N-methyltransferase1. J Med Chem48:1806-12 (2005) [PubMed] Article
Grunewald, GL; Romero, FA; Criscione, KR Nanomolar inhibitors of CNS epinephrine biosynthesis: (R)-(+)-3-fluoromethyl-7-(N-substituted aminosulfonyl)-1,2,3,4-tetrahydroisoquinolines as potent and highly selective inhibitors of phenylethanolamine N-methyltransferase1. J Med Chem48:1806-12 (2005) [PubMed] Article