| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Cholecystokinin receptor type A |
|---|
| Ligand | BDBM50060322 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_306155 (CHEMBL832343) |
|---|
| IC50 | 6850±n/a nM |
|---|
| Citation |  Martín-Martínez, M; Marty, A; Jourdan, M; Escrieut, C; Archer, E; González-Muñiz, R; García-López, MT; Maigret, B; Herranz, R; Fourmy, D Combination of molecular modeling, site-directed mutagenesis, and SAR studies to delineate the binding site of pyridopyrimidine antagonists on the human CCK1 receptor. J Med Chem48:4842-50 (2005) [PubMed] Article Martín-Martínez, M; Marty, A; Jourdan, M; Escrieut, C; Archer, E; González-Muñiz, R; García-López, MT; Maigret, B; Herranz, R; Fourmy, D Combination of molecular modeling, site-directed mutagenesis, and SAR studies to delineate the binding site of pyridopyrimidine antagonists on the human CCK1 receptor. J Med Chem48:4842-50 (2005) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Cholecystokinin receptor type A |
|---|
| Name: | Cholecystokinin receptor type A |
|---|
| Synonyms: | CCK-A receptor | CCK-AR | CCK1-R | CCKAR | CCKAR_HUMAN | CCKRA | Cholecystokinin receptor | Cholecystokinin receptor type A | Cholecystokinin-1 Receptor |
|---|
| Type: | G Protein-Coupled Receptor (GPCR) |
|---|
| Mol. Mass.: | 47859.34 |
|---|
| Organism: | Homo sapiens (Human) |
|---|
| Description: | Stable expression of human CCK-1 receptors in HEK 293 cells. |
|---|
| Residue: | 428 |
|---|
| Sequence: | MDVVDSLLVNGSNITPPCELGLENETLFCLDQPRPSKEWQPAVQILLYSLIFLLSVLGNT
LVITVLIRNKRMRTVTNIFLLSLAVSDLMLCLFCMPFNLIPNLLKDFIFGSAVCKTTTYF
MGTSVSVSTFNLVAISLERYGAICKPLQSRVWQTKSHALKVIAATWCLSFTIMTPYPIYS
NLVPFTKNNNQTANMCRFLLPNDVMQQSWHTFLLLILFLIPGIVMMVAYGLISLELYQGI
KFEASQKKSAKERKPSTTSSGKYEDSDGCYLQKTRPPRKLELRQLSTGSSSRANRIRSNS
SAANLMAKKRVIRMLIVIVVLFFLCWMPIFSANAWRAYDTASAERRLSGTPISFILLLSY
TSSCVNPIIYCFMNKRFRLGFMATFPCCPNPGPPGARGEVGEEEEGGTTGASLSRFSYSH
MSASVPPQ
|
|
|
|---|
| BDBM50060322 |
|---|
| n/a |
|---|
| Name | BDBM50060322 |
|---|
| Synonyms: | CHEMBL332261 | [(S)-1-((4aS,5S)-2-Benzyl-1,3-dioxo-octahydro-pyrido[1,2-c]pyrimidin-5-ylcarbamoyl)-2-(1H-indol-3-yl)-ethyl]-carbamic acid tert-butyl ester |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C31H37N5O5 |
|---|
| Mol. Mass. | 559.656 |
|---|
| SMILES | CC(C)(C)OC(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N[C@H]1CCCN2[C@H]1CC(=O)N(Cc1ccccc1)C2=O |
|---|
| Structure | 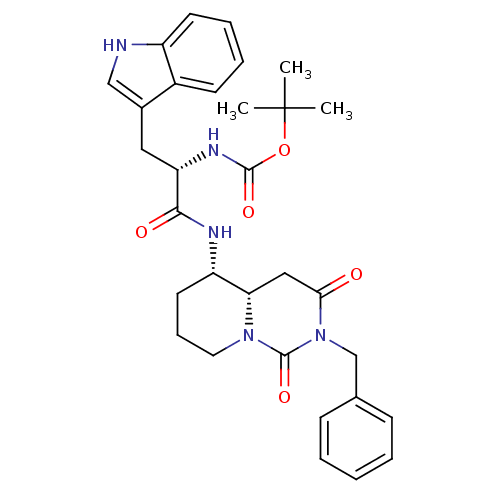
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Martín-Martínez, M; Marty, A; Jourdan, M; Escrieut, C; Archer, E; González-Muñiz, R; García-López, MT; Maigret, B; Herranz, R; Fourmy, D Combination of molecular modeling, site-directed mutagenesis, and SAR studies to delineate the binding site of pyridopyrimidine antagonists on the human CCK1 receptor. J Med Chem48:4842-50 (2005) [PubMed] Article
Martín-Martínez, M; Marty, A; Jourdan, M; Escrieut, C; Archer, E; González-Muñiz, R; García-López, MT; Maigret, B; Herranz, R; Fourmy, D Combination of molecular modeling, site-directed mutagenesis, and SAR studies to delineate the binding site of pyridopyrimidine antagonists on the human CCK1 receptor. J Med Chem48:4842-50 (2005) [PubMed] Article