| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Glycogen synthase kinase-3 beta |
|---|
| Ligand | BDBM50613394 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_2292293 |
|---|
| IC50 | 200±n/a nM |
|---|
| Citation |  Luo, G; Chen, L; Burton, CR; Xiao, H; Sivaprakasam, P; Krause, CM; Cao, Y; Liu, N; Lippy, J; Clarke, WJ; Snow, K; Raybon, J; Arora, V; Pokross, M; Kish, K; Lewis, HA; Langley, DR; Macor, JE; Dubowchik, GM Discovery of Isonicotinamides as Highly Selective, Brain Penetrable, and Orally Active Glycogen Synthase Kinase-3 Inhibitors. J Med Chem59:1041-51 (2016) [PubMed] Luo, G; Chen, L; Burton, CR; Xiao, H; Sivaprakasam, P; Krause, CM; Cao, Y; Liu, N; Lippy, J; Clarke, WJ; Snow, K; Raybon, J; Arora, V; Pokross, M; Kish, K; Lewis, HA; Langley, DR; Macor, JE; Dubowchik, GM Discovery of Isonicotinamides as Highly Selective, Brain Penetrable, and Orally Active Glycogen Synthase Kinase-3 Inhibitors. J Med Chem59:1041-51 (2016) [PubMed] |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Glycogen synthase kinase-3 beta |
|---|
| Name: | Glycogen synthase kinase-3 beta |
|---|
| Synonyms: | GSK-3 beta | GSK-3, beta | GSK3B | GSK3B_HUMAN | Glycogen synthase kinase 3 beta (GSK3B) | Glycogen synthase kinase 3-beta (GSK3B) | Glycogen synthase kinase-3 beta (GSK-3B) | Glycogen synthase kinase-3 beta (GSK3 Beta) | Glycogen synthase kinase-3 beta (GSK3B) | Glycogen synthase kinase-3B (GSK-3B) | Glycogen synthase kinase-3beta (GSK3B) | Serine/threonine-protein kinase GSK3B |
|---|
| Type: | Enzyme |
|---|
| Mol. Mass.: | 46756.38 |
|---|
| Organism: | Homo sapiens (Human) |
|---|
| Description: | P49841 |
|---|
| Residue: | 420 |
|---|
| Sequence: | MSGRPRTTSFAESCKPVQQPSAFGSMKVSRDKDGSKVTTVVATPGQGPDRPQEVSYTDTK
VIGNGSFGVVYQAKLCDSGELVAIKKVLQDKRFKNRELQIMRKLDHCNIVRLRYFFYSSG
EKKDEVYLNLVLDYVPETVYRVARHYSRAKQTLPVIYVKLYMYQLFRSLAYIHSFGICHR
DIKPQNLLLDPDTAVLKLCDFGSAKQLVRGEPNVSYICSRYYRAPELIFGATDYTSSIDV
WSAGCVLAELLLGQPIFPGDSGVDQLVEIIKVLGTPTREQIREMNPNYTEFKFPQIKAHP
WTKVFRPRTPPEAIALCSRLLEYTPTARLTPLEACAHSFFDELRDPNVKLPNGRDTPALF
NFTTQELSSNPPLATILIPPHARIQAAASTPTNATAASDANTGDRGQTNNAASASASNST
|
|
|
|---|
| BDBM50613394 |
|---|
| n/a |
|---|
| Name | BDBM50613394 |
|---|
| Synonyms: | CHEMBL5278465 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C21H17ClN4O2 |
|---|
| Mol. Mass. | 392.838 |
|---|
| SMILES | Clc1ccc(cc1)-c1ccncc1NC(=O)c1ccnc(NC(=O)C2CC2)c1 |
|---|
| Structure | 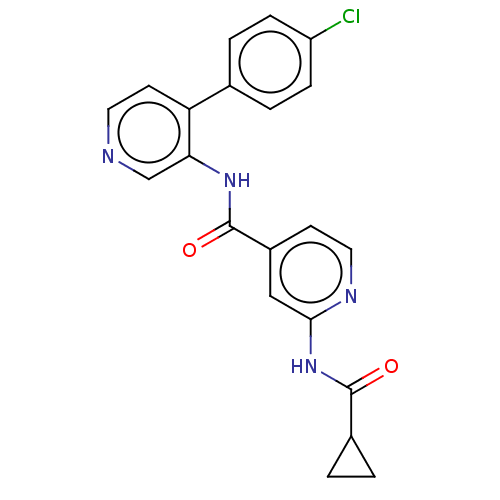
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Luo, G; Chen, L; Burton, CR; Xiao, H; Sivaprakasam, P; Krause, CM; Cao, Y; Liu, N; Lippy, J; Clarke, WJ; Snow, K; Raybon, J; Arora, V; Pokross, M; Kish, K; Lewis, HA; Langley, DR; Macor, JE; Dubowchik, GM Discovery of Isonicotinamides as Highly Selective, Brain Penetrable, and Orally Active Glycogen Synthase Kinase-3 Inhibitors. J Med Chem59:1041-51 (2016) [PubMed]
Luo, G; Chen, L; Burton, CR; Xiao, H; Sivaprakasam, P; Krause, CM; Cao, Y; Liu, N; Lippy, J; Clarke, WJ; Snow, K; Raybon, J; Arora, V; Pokross, M; Kish, K; Lewis, HA; Langley, DR; Macor, JE; Dubowchik, GM Discovery of Isonicotinamides as Highly Selective, Brain Penetrable, and Orally Active Glycogen Synthase Kinase-3 Inhibitors. J Med Chem59:1041-51 (2016) [PubMed]