

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Reaction Details | |||
|---|---|---|---|
 | Report a problem with these data | ||
| Target | Melanocortin receptor 3 | ||
| Ligand | BDBM50217678 | ||
| Substrate/Competitor | n/a | ||
| Meas. Tech. | ChEMBL_448691 (CHEMBL896691) | ||
| Ki | 27000±n/a nM | ||
| Citation |  Mutulis, F; Kreicberga, J; Yahorava, S; Mutule, I; Borisova-Jan, L; Yahorau, A; Muceniece, R; Azena, S; Veiksina, S; Petrovska, R; Wikberg, JE Design and synthesis of a library of tertiary amides: evaluation as mimetics of the melanocortins' active core. Bioorg Med Chem15:5787-810 (2007) [PubMed] Article Mutulis, F; Kreicberga, J; Yahorava, S; Mutule, I; Borisova-Jan, L; Yahorau, A; Muceniece, R; Azena, S; Veiksina, S; Petrovska, R; Wikberg, JE Design and synthesis of a library of tertiary amides: evaluation as mimetics of the melanocortins' active core. Bioorg Med Chem15:5787-810 (2007) [PubMed] Article | ||
| More Info.: | Get all data from this article, Assay Method | ||
| Melanocortin receptor 3 | |||
| Name: | Melanocortin receptor 3 | ||
| Synonyms: | MC3-R | MC3R | MC3R_HUMAN | Melanocortin MC3 | Melanocortin receptor (M3 and M4) | ||
| Type: | Enzyme | ||
| Mol. Mass.: | 36044.86 | ||
| Organism: | Homo sapiens (Human) | ||
| Description: | P41968 | ||
| Residue: | 323 | ||
| Sequence: |
| ||
| BDBM50217678 | |||
| n/a | |||
| Name | BDBM50217678 | ||
| Synonyms: | CHEMBL235582 | N-(4-((4-aminocyclohexyl)methyl)cyclohexyl)-N-(2-bromoallyl)-4-(1H-indol-3-yl)butanamide | ||
| Type | Small organic molecule | ||
| Emp. Form. | C28H40BrN3O | ||
| Mol. Mass. | 514.541 | ||
| SMILES | NC1CCC(CC2CCC(CC2)N(CC(Br)=C)C(=O)CCCc2c[nH]c3ccccc23)CC1 |(20.22,4.36,;18.9,3.59,;18.91,2.05,;17.59,1.27,;16.25,2.03,;14.92,1.25,;14.94,-.29,;16.29,-1.05,;16.31,-2.61,;14.97,-3.39,;13.61,-2.65,;13.61,-1.08,;15,-4.95,;13.64,-5.91,;12.13,-5.21,;12.12,-3.54,;10.77,-6.18,;16.35,-5.71,;16.38,-7.26,;17.68,-4.91,;19.02,-5.65,;20.36,-4.88,;21.68,-5.64,;23.09,-4.97,;24.14,-6.09,;23.39,-7.44,;23.88,-8.88,;22.88,-10.04,;21.37,-9.74,;20.88,-8.29,;21.89,-7.13,;16.23,3.56,;17.56,4.34,)| | ||
| Structure | 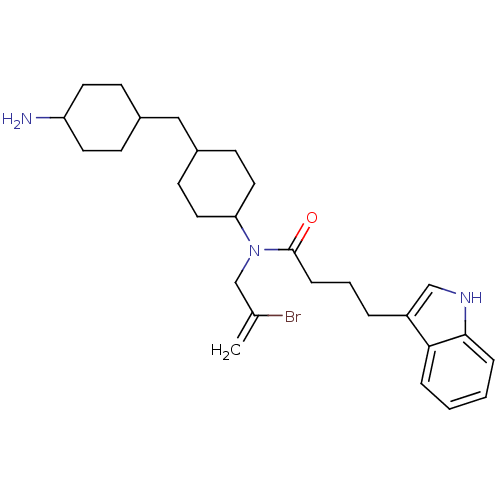
| ||