| Reaction Details |
|---|
 | Report a problem with these data |
| Target | C-C chemokine receptor type 5 |
|---|
| Ligand | BDBM50121828 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_500129 (CHEMBL974332) |
|---|
| IC50 | 0.2±n/a nM |
|---|
| Citation |  Zhuo, Y; Kong, R; Cong, XJ; Chen, WZ; Wang, CX Three-dimensional QSAR analyses of 1,3,4-trisubstituted pyrrolidine-based CCR5 receptor inhibitors. Eur J Med Chem43:2724-34 (2008) [PubMed] Article Zhuo, Y; Kong, R; Cong, XJ; Chen, WZ; Wang, CX Three-dimensional QSAR analyses of 1,3,4-trisubstituted pyrrolidine-based CCR5 receptor inhibitors. Eur J Med Chem43:2724-34 (2008) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| C-C chemokine receptor type 5 |
|---|
| Name: | C-C chemokine receptor type 5 |
|---|
| Synonyms: | C-C CKR-5 | C-C chemokine receptor type 5 | CC-CKR-5 | CCR-5 | CCR5 | CCR5/mu opioid receptor complex | CCR5_HUMAN | CD_antigen=CD195 | CHEMR13 | CMKBR5 | HIV-1 fusion coreceptor |
|---|
| Type: | Enzyme |
|---|
| Mol. Mass.: | 40540.21 |
|---|
| Organism: | Homo sapiens (Human) |
|---|
| Description: | P51681 |
|---|
| Residue: | 352 |
|---|
| Sequence: | MDYQVSSPIYDINYYTSEPCQKINVKQIAARLLPPLYSLVFIFGFVGNMLVILILINCKR
LKSMTDIYLLNLAISDLFFLLTVPFWAHYAAAQWDFGNTMCQLLTGLYFIGFFSGIFFII
LLTIDRYLAVVHAVFALKARTVTFGVVTSVITWVVAVFASLPGIIFTRSQKEGLHYTCSS
HFPYSQYQFWKNFQTLKIVILGLVLPLLVMVICYSGILKTLLRCRNEKKRHRAVRLIFTI
MIVYFLFWAPYNIVLLLNTFQEFFGLNNCSSSNRLDQAMQVTETLGMTHCCINPIIYAFV
GEKFRNYLLVFFQKHIAKRFCKCCSIFQQEAPERASSVYTRSTGEQEISVGL
|
|
|
|---|
| BDBM50121828 |
|---|
| n/a |
|---|
| Name | BDBM50121828 |
|---|
| Synonyms: | (R)-3-Cyclobutyl-2-((S)-3-(3-fluoro-phenyl)-4-{4-[3-(4-trifluoromethyl-phenyl)-propyl]-piperidin-1-ylmethyl}-pyrrolidin-1-yl)-propionic acid | (R)-3-cyclobutyl-2-((3S,4S)-3-(3-fluorophenyl)-4-((4-(3-(4-(trifluoromethyl)phenyl)propyl)piperidin-1-yl)methyl)pyrrolidin-1-yl)propanoic acid | CHEMBL21147 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C33H42F4N2O2 |
|---|
| Mol. Mass. | 574.6924 |
|---|
| SMILES | OC(=O)[C@@H](CC1CCC1)N1C[C@H](CN2CCC(CCCc3ccc(cc3)C(F)(F)F)CC2)[C@H](C1)c1cccc(F)c1 |r| |
|---|
| Structure | 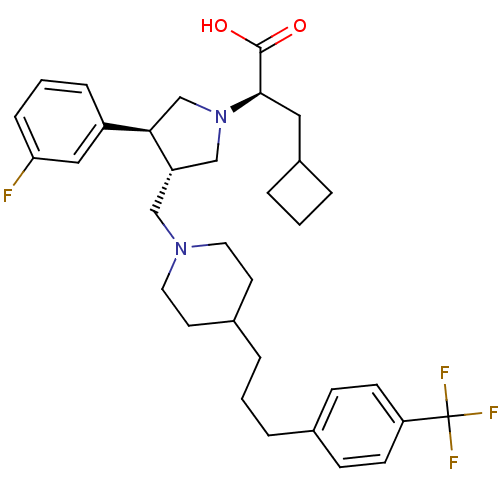
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Zhuo, Y; Kong, R; Cong, XJ; Chen, WZ; Wang, CX Three-dimensional QSAR analyses of 1,3,4-trisubstituted pyrrolidine-based CCR5 receptor inhibitors. Eur J Med Chem43:2724-34 (2008) [PubMed] Article
Zhuo, Y; Kong, R; Cong, XJ; Chen, WZ; Wang, CX Three-dimensional QSAR analyses of 1,3,4-trisubstituted pyrrolidine-based CCR5 receptor inhibitors. Eur J Med Chem43:2724-34 (2008) [PubMed] Article