| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Peroxisome proliferator-activated receptor alpha |
|---|
| Ligand | BDBM50263955 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_535520 (CHEMBL986146) |
|---|
| EC50 | 3860±n/a nM |
|---|
| Citation |  Kim, E; Park, CS; Han, T; Bae, MH; Chong, W; Lee, CH; Shin, YA; Ahn, BN; Kim, MK; Shin, CY; Son, MH; Kim, JK; Moon, HS; Shim, HJ; Kim, EJ; Kim, SH; Lim, JI; Lee, CH Design, synthesis, and evaluation of novel aryl-tetrahydropyridine PPARalpha/gamma dual agonists. Bioorg Med Chem Lett18:4993-6 (2008) [PubMed] Article Kim, E; Park, CS; Han, T; Bae, MH; Chong, W; Lee, CH; Shin, YA; Ahn, BN; Kim, MK; Shin, CY; Son, MH; Kim, JK; Moon, HS; Shim, HJ; Kim, EJ; Kim, SH; Lim, JI; Lee, CH Design, synthesis, and evaluation of novel aryl-tetrahydropyridine PPARalpha/gamma dual agonists. Bioorg Med Chem Lett18:4993-6 (2008) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Peroxisome proliferator-activated receptor alpha |
|---|
| Name: | Peroxisome proliferator-activated receptor alpha |
|---|
| Synonyms: | NR1C1 | Nuclear receptor subfamily 1 group C member 1 | PPAR | PPAR alpha/gamma | PPAR-alpha | PPARA | PPARA_HUMAN | Peroxisome Proliferator-Activated Receptor alpha | Peroxisome proliferator-activated receptor | Peroxisome proliferator-activated receptor alpha (PPAR alpha) |
|---|
| Type: | Enzyme |
|---|
| Mol. Mass.: | 52222.08 |
|---|
| Organism: | Homo sapiens (Human) |
|---|
| Description: | Q07869 |
|---|
| Residue: | 468 |
|---|
| Sequence: | MVDTESPLCPLSPLEAGDLESPLSEEFLQEMGNIQEISQSIGEDSSGSFGFTEYQYLGSC
PGSDGSVITDTLSPASSPSSVTYPVVPGSVDESPSGALNIECRICGDKASGYHYGVHACE
GCKGFFRRTIRLKLVYDKCDRSCKIQKKNRNKCQYCRFHKCLSVGMSHNAIRFGRMPRSE
KAKLKAEILTCEHDIEDSETADLKSLAKRIYEAYLKNFNMNKVKARVILSGKASNNPPFV
IHDMETLCMAEKTLVAKLVANGIQNKEAEVRIFHCCQCTSVETVTELTEFAKAIPGFANL
DLNDQVTLLKYGVYEAIFAMLSSVMNKDGMLVAYGNGFITREFLKSLRKPFCDIMEPKFD
FAMKFNALELDDSDISLFVAAIICCGDRPGLLNVGHIEKMQEGIVHVLRLHLQSNHPDDI
FLFPKLLQKMADLRQLVTEHAQLVQIIKKTESDAALHPLLQEIYRDMY
|
|
|
|---|
| BDBM50263955 |
|---|
| n/a |
|---|
| Name | BDBM50263955 |
|---|
| Synonyms: | 2-(4-(2-(4-(4-chlorophenyl)-5,6-dihydropyridin-1(2H)-yl)ethoxy)benzyl)butanoic acid | CHEMBL491596 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C24H28ClNO3 |
|---|
| Mol. Mass. | 413.937 |
|---|
| SMILES | CCC(Cc1ccc(OCCN2CCC(=CC2)c2ccc(Cl)cc2)cc1)C(O)=O |c:14| |
|---|
| Structure | 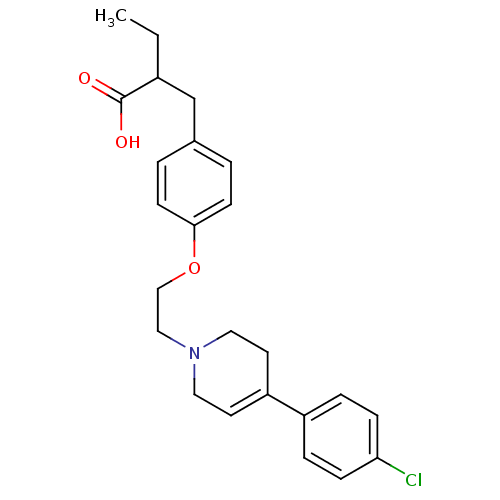
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Kim, E; Park, CS; Han, T; Bae, MH; Chong, W; Lee, CH; Shin, YA; Ahn, BN; Kim, MK; Shin, CY; Son, MH; Kim, JK; Moon, HS; Shim, HJ; Kim, EJ; Kim, SH; Lim, JI; Lee, CH Design, synthesis, and evaluation of novel aryl-tetrahydropyridine PPARalpha/gamma dual agonists. Bioorg Med Chem Lett18:4993-6 (2008) [PubMed] Article
Kim, E; Park, CS; Han, T; Bae, MH; Chong, W; Lee, CH; Shin, YA; Ahn, BN; Kim, MK; Shin, CY; Son, MH; Kim, JK; Moon, HS; Shim, HJ; Kim, EJ; Kim, SH; Lim, JI; Lee, CH Design, synthesis, and evaluation of novel aryl-tetrahydropyridine PPARalpha/gamma dual agonists. Bioorg Med Chem Lett18:4993-6 (2008) [PubMed] Article