| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Lysosomal acid glucosylceramidase |
|---|
| Ligand | BDBM50280031 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_218239 (CHEMBL823892) |
|---|
| pH | 5±n/a |
|---|
| Ki | 900±n/a nM |
|---|
| Comments | extracted |
|---|
| Citation |  Berger, A; Dax, K; Gradnig, G; Grassberger, V; Stütz, AE; Ungerank, M; Legler, G; Bause, E Synthesis and biological activity of C-6 modified derivatives of the glucosidase inhibitor 1-deoxynojirimycin. Bioorg Med Chem Lett2:27-32 (1992) Article Berger, A; Dax, K; Gradnig, G; Grassberger, V; Stütz, AE; Ungerank, M; Legler, G; Bause, E Synthesis and biological activity of C-6 modified derivatives of the glucosidase inhibitor 1-deoxynojirimycin. Bioorg Med Chem Lett2:27-32 (1992) Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Lysosomal acid glucosylceramidase |
|---|
| Name: | Lysosomal acid glucosylceramidase |
|---|
| Synonyms: | Acid beta-glucosidase | Alglucerase | Beta-glucocerebrosidase | Beta-glucocerebrosidase (GC) | D-glucosyl-N-acylsphingosine glucohydrolase | GBA | GBA1 | GBA1_HUMAN | GC | GCase | GLUC | Glucocerebrosidase (GBA) | Glucosylceramidase (GBA) | Glucosylceramidase (GCase) | Glucosylceramidase precursor (Beta-glucocerebrosidase) (Acid beta-glucosidase) (D-glucosyl-N-acylsphingosine glucohydrolase) (Alglucerase) (Imiglucerase) | Imiglucerase | beta-glucocerebrosidase (GCase) |
|---|
| Type: | Enzyme |
|---|
| Mol. Mass.: | 59724.64 |
|---|
| Organism: | Homo sapiens (Human) |
|---|
| Description: | The beta-Glu activity was measured with commercially available beta-glucocerebrosidase (Ceredase) as the enzyme source. |
|---|
| Residue: | 536 |
|---|
| Sequence: | MEFSSPSREECPKPLSRVSIMAGSLTGLLLLQAVSWASGARPCIPKSFGYSSVVCVCNAT
YCDSFDPPTFPALGTFSRYESTRSGRRMELSMGPIQANHTGTGLLLTLQPEQKFQKVKGF
GGAMTDAAALNILALSPPAQNLLLKSYFSEEGIGYNIIRVPMASCDFSIRTYTYADTPDD
FQLHNFSLPEEDTKLKIPLIHRALQLAQRPVSLLASPWTSPTWLKTNGAVNGKGSLKGQP
GDIYHQTWARYFVKFLDAYAEHKLQFWAVTAENEPSAGLLSGYPFQCLGFTPEHQRDFIA
RDLGPTLANSTHHNVRLLMLDDQRLLLPHWAKVVLTDPEAAKYVHGIAVHWYLDFLAPAK
ATLGETHRLFPNTMLFASEACVGSKFWEQSVRLGSWDRGMQYSHSIITNLLYHVVGWTDW
NLALNPEGGPNWVRNFVDSPIIVDITKDTFYKQPMFYHLGHFSKFIPEGSQRVGLVASQK
NDLDAVALMHPDGSAVVVVLNRSSKDVPLTIKDPAVGFLETISPGYSIHTYLWRRQ
|
|
|
|---|
| BDBM50280031 |
|---|
| n/a |
|---|
| Name | BDBM50280031 |
|---|
| Synonyms: | (6S,7R,8R)-Octahydro-indolizine-1,6,7,8-tetraol | CHEMBL421040 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C8H15NO4 |
|---|
| Mol. Mass. | 189.209 |
|---|
| SMILES | OC1CCN2C[C@H](O)[C@@H](O)[C@H](O)C12 |
|---|
| Structure | 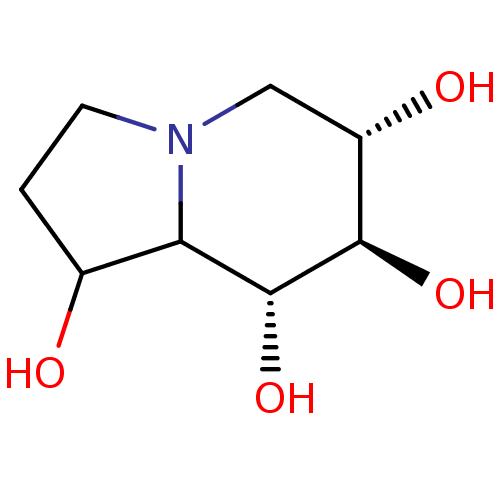
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Berger, A; Dax, K; Gradnig, G; Grassberger, V; Stütz, AE; Ungerank, M; Legler, G; Bause, E Synthesis and biological activity of C-6 modified derivatives of the glucosidase inhibitor 1-deoxynojirimycin. Bioorg Med Chem Lett2:27-32 (1992) Article
Berger, A; Dax, K; Gradnig, G; Grassberger, V; Stütz, AE; Ungerank, M; Legler, G; Bause, E Synthesis and biological activity of C-6 modified derivatives of the glucosidase inhibitor 1-deoxynojirimycin. Bioorg Med Chem Lett2:27-32 (1992) Article