| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Adenosine receptor A1 |
|---|
| Ligand | BDBM50004566 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_29476 (CHEMBL640469) |
|---|
| Ki | 6.4±n/a nM |
|---|
| Citation |  Baraldi, PG; Manfredini, S; Simoni, D; Zappaterra, L; Zocchi, C; Dionisotti, S; Ongini, E Synthesis of new pyrazolo[4,3-e]1,2,4-triazolo[1,5-c] pyrimidine and 1,2,3-triazolo[4,5-e]1,2,4-triazolo[1,5-c] pyrimidine displaying potent and selective activity as A2a adenosine receptor antagonists. Bioorg Med Chem Lett4:2539-2544 (1994) Article Baraldi, PG; Manfredini, S; Simoni, D; Zappaterra, L; Zocchi, C; Dionisotti, S; Ongini, E Synthesis of new pyrazolo[4,3-e]1,2,4-triazolo[1,5-c] pyrimidine and 1,2,3-triazolo[4,5-e]1,2,4-triazolo[1,5-c] pyrimidine displaying potent and selective activity as A2a adenosine receptor antagonists. Bioorg Med Chem Lett4:2539-2544 (1994) Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Adenosine receptor A1 |
|---|
| Name: | Adenosine receptor A1 |
|---|
| Synonyms: | AA1R_RAT | ADENOSINE A1 | ADENOSINE A1 high | ADENOSINE A1 low | Adenosine A1 receptor (A1) | Adenosine receptor | Adenosine receptors A1 | Adora1 |
|---|
| Type: | Protein |
|---|
| Mol. Mass.: | 36704.13 |
|---|
| Organism: | Rattus norvegicus (rat) |
|---|
| Description: | n/a |
|---|
| Residue: | 326 |
|---|
| Sequence: | MPPYISAFQAAYIGIEVLIALVSVPGNVLVIWAVKVNQALRDATFCFIVSLAVADVAVGA
LVIPLAILINIGPQTYFHTCLMVACPVLILTQSSILALLAIAVDRYLRVKIPLRYKTVVT
QRRAAVAIAGCWILSLVVGLTPMFGWNNLSVVEQDWRANGSVGEPVIKCEFEKVISMEYM
VYFNFFVWVLPPLLLMVLIYLEVFYLIRKQLNKKVSASSGDPQKYYGKELKIAKSLALIL
FLFALSWLPLHILNCITLFCPTCQKPSILIYIAIFLTHGNSAMNPIVYAFRIHKFRVTFL
KIWNDHFRCQPKPPIDEDLPEEKAED
|
|
|
|---|
| BDBM50004566 |
|---|
| n/a |
|---|
| Name | BDBM50004566 |
|---|
| Synonyms: | 9-Chloro-2-furan-2-yl-[1,2,4]triazolo[1,5-c]quinazolin-5-ylamine | 9-Chloro-2-furan-2-yl-[1,2,4]triazolo[1,5-c]quinazolin-5-ylamine(CGS 15943) | 9-Chloro-[1,2,4]triazolo[1,5-c]quinazolin-5-ylamine(CGS 15943) | 9-chloro-2-(2-furanyl)-1,2,4-triazolo[1.5-c]quinazolin-5-amine | 9-chloro-2-(furan-2-yl)-[1,2,4]triazolo[1,5-c]quinazolin-5-amine | CGS-15943 | CHEMBL16687 | CHEMBL268431 | Nonnucleoside analog, 4 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C13H8ClN5O |
|---|
| Mol. Mass. | 285.689 |
|---|
| SMILES | Nc1nc2ccc(Cl)cc2c2nc(nn12)-c1ccco1 |
|---|
| Structure | 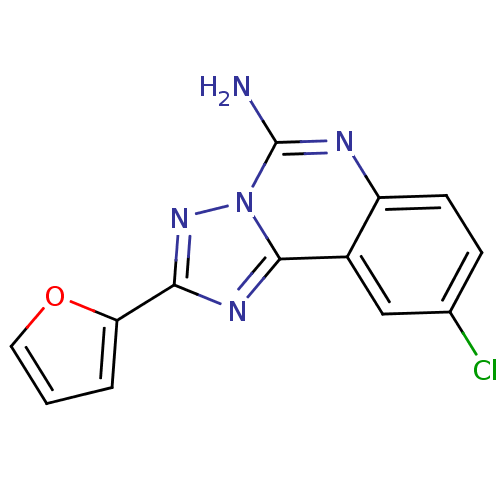
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Baraldi, PG; Manfredini, S; Simoni, D; Zappaterra, L; Zocchi, C; Dionisotti, S; Ongini, E Synthesis of new pyrazolo[4,3-e]1,2,4-triazolo[1,5-c] pyrimidine and 1,2,3-triazolo[4,5-e]1,2,4-triazolo[1,5-c] pyrimidine displaying potent and selective activity as A2a adenosine receptor antagonists. Bioorg Med Chem Lett4:2539-2544 (1994) Article
Baraldi, PG; Manfredini, S; Simoni, D; Zappaterra, L; Zocchi, C; Dionisotti, S; Ongini, E Synthesis of new pyrazolo[4,3-e]1,2,4-triazolo[1,5-c] pyrimidine and 1,2,3-triazolo[4,5-e]1,2,4-triazolo[1,5-c] pyrimidine displaying potent and selective activity as A2a adenosine receptor antagonists. Bioorg Med Chem Lett4:2539-2544 (1994) Article