| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Endothelin-1 receptor |
|---|
| Ligand | BDBM50098772 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEBML_65629 |
|---|
| IC50 | 630±n/a nM |
|---|
| Citation |  Raju, B; Okun, I; Stavros, F; Chan, MF Amide bond surrogates: A study in thiophenesulfonamide based endothelin receptor antagonists Bioorg Med Chem Lett7:939-944 (1997) Article Raju, B; Okun, I; Stavros, F; Chan, MF Amide bond surrogates: A study in thiophenesulfonamide based endothelin receptor antagonists Bioorg Med Chem Lett7:939-944 (1997) Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Endothelin-1 receptor |
|---|
| Name: | Endothelin-1 receptor |
|---|
| Synonyms: | EDNRA | EDNRA_HUMAN | ET-A | ETA | ETA-R | ETRA | Endothelin receptor type A | Endothelin receptor, ET-A/ET-B | hET-AR |
|---|
| Type: | Enzyme Catalytic Domain |
|---|
| Mol. Mass.: | 48736.88 |
|---|
| Organism: | Homo sapiens (Human) |
|---|
| Description: | P25101 |
|---|
| Residue: | 427 |
|---|
| Sequence: | METLCLRASFWLALVGCVISDNPERYSTNLSNHVDDFTTFRGTELSFLVTTHQPTNLVLP
SNGSMHNYCPQQTKITSAFKYINTVISCTIFIVGMVGNATLLRIIYQNKCMRNGPNALIA
SLALGDLIYVVIDLPINVFKLLAGRWPFDHNDFGVFLCKLFPFLQKSSVGITVLNLCALS
VDRYRAVASWSRVQGIGIPLVTAIEIVSIWILSFILAIPEAIGFVMVPFEYRGEQHKTCM
LNATSKFMEFYQDVKDWWLFGFYFCMPLVCTAIFYTLMTCEMLNRRNGSLRIALSEHLKQ
RREVAKTVFCLVVIFALCWFPLHLSRILKKTVYNEMDKNRCELLSFLLLMDYIGINLATM
NSCINPIALYFVSKKFKNCFQSCLCCCCYQSKSLMTSVPMNGTSIQWKNHDQNNHNTDRS
SHKDSMN
|
|
|
|---|
| BDBM50098772 |
|---|
| n/a |
|---|
| Name | BDBM50098772 |
|---|
| Synonyms: | 3-(4-Chloro-3-methyl-isoxazol-5-ylsulfamoyl)-thiophene-2-carboxylic acid (2,4,6-trimethyl-phenyl)-amide | CHEMBL277595 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C18H18ClN3O4S2 |
|---|
| Mol. Mass. | 439.936 |
|---|
| SMILES | Cc1noc(NS(=O)(=O)c2ccsc2C(=O)Nc2c(C)cc(C)cc2C)c1Cl |
|---|
| Structure | 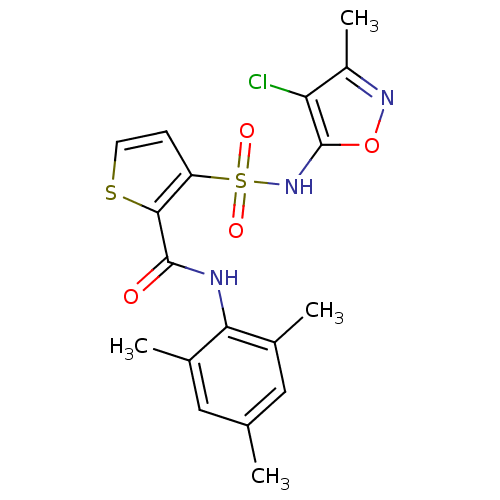
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Raju, B; Okun, I; Stavros, F; Chan, MF Amide bond surrogates: A study in thiophenesulfonamide based endothelin receptor antagonists Bioorg Med Chem Lett7:939-944 (1997) Article
Raju, B; Okun, I; Stavros, F; Chan, MF Amide bond surrogates: A study in thiophenesulfonamide based endothelin receptor antagonists Bioorg Med Chem Lett7:939-944 (1997) Article