| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Histamine H3 receptor |
|---|
| Ligand | BDBM50293632 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_571442 (CHEMBL1032753) |
|---|
| IC50 | 2110±n/a nM |
|---|
| Citation |  Kennedy, JP; Conn, PJ; Lindsley, CW A novel class of H3 antagonists derived from the natural product guided synthesis of unnatural analogs of the marine bromopyrrole alkaloid dispyrin. Bioorg Med Chem Lett19:3204-8 (2009) [PubMed] Article Kennedy, JP; Conn, PJ; Lindsley, CW A novel class of H3 antagonists derived from the natural product guided synthesis of unnatural analogs of the marine bromopyrrole alkaloid dispyrin. Bioorg Med Chem Lett19:3204-8 (2009) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Histamine H3 receptor |
|---|
| Name: | Histamine H3 receptor |
|---|
| Synonyms: | G-protein coupled receptor 97 | GPCR97 | HH3R | HISTAMINE H3 | HRH3 | HRH3_HUMAN | Histamine H3 receptor (H3) | Histamine H3L | Histamine receptor (H3 and H4) |
|---|
| Type: | G Protein-Coupled Receptor (GPCR) |
|---|
| Mol. Mass.: | 48691.47 |
|---|
| Organism: | Homo sapiens (Human) |
|---|
| Description: | Binding assays were using CHO cells stably expressing hH3R receptors. |
|---|
| Residue: | 445 |
|---|
| Sequence: | MERAPPDGPLNASGALAGEAAAAGGARGFSAAWTAVLAALMALLIVATVLGNALVMLAFV
ADSSLRTQNNFFLLNLAISDFLVGAFCIPLYVPYVLTGRWTFGRGLCKLWLVVDYLLCTS
SAFNIVLISYDRFLSVTRAVSYRAQQGDTRRAVRKMLLVWVLAFLLYGPAILSWEYLSGG
SSIPEGHCYAEFFYNWYFLITASTLEFFTPFLSVTFFNLSIYLNIQRRTRLRLDGAREAA
GPEPPPEAQPSPPPPPGCWGCWQKGHGEAMPLHRYGVGEAAVGAEAGEATLGGGGGGGSV
ASPTSSSGSSSRGTERPRSLKRGSKPSASSASLEKRMKMVSQSFTQRFRLSRDRKVAKSL
AVIVSIFGLCWAPYTLLMIIRAACHGHCVPDYWYETSFWLLWANSAVNPVLYPLCHHSFR
RAFTKLLCPQKLKIQPHSSLEHCWK
|
|
|
|---|
| BDBM50293632 |
|---|
| n/a |
|---|
| Name | BDBM50293632 |
|---|
| Synonyms: | 4-bromo-N-(3-bromo-4-(2-(3-fluoropyrrolidin-1-yl)ethoxy)phenethyl)-4-bromothiophene-2-carboxamide | 4-bromo-N-(3-bromo-4-(2-(3-fluoropyrrolidin-1-yl)ethoxy)phenethyl)thiophene-2-carboxamide | CHEMBL562496 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C19H21Br2FN2O2S |
|---|
| Mol. Mass. | 520.254 |
|---|
| SMILES | FC1CCN(CCOc2ccc(CCNC(=O)c3cc(Br)cs3)cc2Br)C1 |
|---|
| Structure | 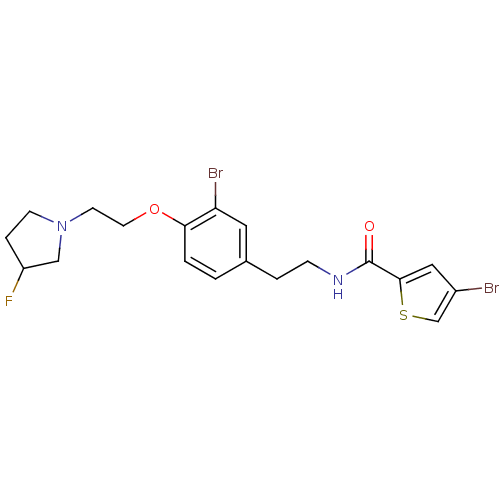
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Kennedy, JP; Conn, PJ; Lindsley, CW A novel class of H3 antagonists derived from the natural product guided synthesis of unnatural analogs of the marine bromopyrrole alkaloid dispyrin. Bioorg Med Chem Lett19:3204-8 (2009) [PubMed] Article
Kennedy, JP; Conn, PJ; Lindsley, CW A novel class of H3 antagonists derived from the natural product guided synthesis of unnatural analogs of the marine bromopyrrole alkaloid dispyrin. Bioorg Med Chem Lett19:3204-8 (2009) [PubMed] Article