| Reaction Details |
|---|
 | Report a problem with these data |
| Target | 5-hydroxytryptamine receptor 1A |
|---|
| Ligand | BDBM50295064 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_574193 (CHEMBL1060394) |
|---|
| IC50 | 781±n/a nM |
|---|
| Citation |  Zhou, D; Stack, GP; Lo, J; Failli, AA; Evrard, DA; Harrison, BL; Hatzenbuhler, NT; Tran, M; Croce, S; Yi, S; Golembieski, J; Hornby, GA; Lai, M; Lin, Q; Schechter, LE; Smith, DL; Shilling, AD; Huselton, C; Mitchell, P; Beyer, CE; Andree, TH Synthesis, potency, and in vivo evaluation of 2-piperazin-1-ylquinoline analogues as dual serotonin reuptake inhibitors and serotonin 5-HT1A receptor antagonists. J Med Chem52:4955-9 (2009) [PubMed] Article Zhou, D; Stack, GP; Lo, J; Failli, AA; Evrard, DA; Harrison, BL; Hatzenbuhler, NT; Tran, M; Croce, S; Yi, S; Golembieski, J; Hornby, GA; Lai, M; Lin, Q; Schechter, LE; Smith, DL; Shilling, AD; Huselton, C; Mitchell, P; Beyer, CE; Andree, TH Synthesis, potency, and in vivo evaluation of 2-piperazin-1-ylquinoline analogues as dual serotonin reuptake inhibitors and serotonin 5-HT1A receptor antagonists. J Med Chem52:4955-9 (2009) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| 5-hydroxytryptamine receptor 1A |
|---|
| Name: | 5-hydroxytryptamine receptor 1A |
|---|
| Synonyms: | 5-HT-1A | 5-HT1A | 5-hydroxytryptamine receptor 1A (5-HT-1A) | 5HT1A_HUMAN | ADRB2RL1 | ADRBRL1 | Dopamine D2 receptor and serotonin 1a receptor | G-21 | HTR1A | Serotonin receptor 1A |
|---|
| Type: | n/a |
|---|
| Mol. Mass.: | 46122.49 |
|---|
| Organism: | Homo sapiens (Human) |
|---|
| Description: | n/a |
|---|
| Residue: | 422 |
|---|
| Sequence: | MDVLSPGQGNNTTSPPAPFETGGNTTGISDVTVSYQVITSLLLGTLIFCAVLGNACVVAA
IALERSLQNVANYLIGSLAVTDLMVSVLVLPMAALYQVLNKWTLGQVTCDLFIALDVLCC
TSSILHLCAIALDRYWAITDPIDYVNKRTPRRAAALISLTWLIGFLISIPPMLGWRTPED
RSDPDACTISKDHGYTIYSTFGAFYIPLLLMLVLYGRIFRAARFRIRKTVKKVEKTGADT
RHGASPAPQPKKSVNGESGSRNWRLGVESKAGGALCANGAVRQGDDGAALEVIEVHRVGN
SKEHLPLPSEAGPTPCAPASFERKNERNAEAKRKMALARERKTVKTLGIIMGTFILCWLP
FFIVALVLPFCESSCHMPTLLGAIINWLGYSNSLLNPVIYAYFNKDFQNAFKKIIKCKFC
RQ
|
|
|
|---|
| BDBM50295064 |
|---|
| n/a |
|---|
| Name | BDBM50295064 |
|---|
| Synonyms: | (2S)-2-{[4-(6-Chloroquinolin-2-yl)piperazin-1-yl]methyl}-8-methyl-2,3-dihydro[1,4]dioxino[2,3-f]quinoline | CHEMBL559579 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C26H25ClN4O2 |
|---|
| Mol. Mass. | 460.955 |
|---|
| SMILES | Cc1ccc2c3O[C@@H](CN4CCN(CC4)c4ccc5cc(Cl)ccc5n4)COc3ccc2n1 |r| |
|---|
| Structure | 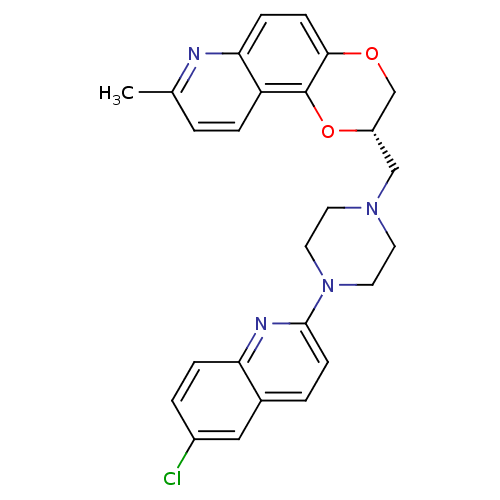
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Zhou, D; Stack, GP; Lo, J; Failli, AA; Evrard, DA; Harrison, BL; Hatzenbuhler, NT; Tran, M; Croce, S; Yi, S; Golembieski, J; Hornby, GA; Lai, M; Lin, Q; Schechter, LE; Smith, DL; Shilling, AD; Huselton, C; Mitchell, P; Beyer, CE; Andree, TH Synthesis, potency, and in vivo evaluation of 2-piperazin-1-ylquinoline analogues as dual serotonin reuptake inhibitors and serotonin 5-HT1A receptor antagonists. J Med Chem52:4955-9 (2009) [PubMed] Article
Zhou, D; Stack, GP; Lo, J; Failli, AA; Evrard, DA; Harrison, BL; Hatzenbuhler, NT; Tran, M; Croce, S; Yi, S; Golembieski, J; Hornby, GA; Lai, M; Lin, Q; Schechter, LE; Smith, DL; Shilling, AD; Huselton, C; Mitchell, P; Beyer, CE; Andree, TH Synthesis, potency, and in vivo evaluation of 2-piperazin-1-ylquinoline analogues as dual serotonin reuptake inhibitors and serotonin 5-HT1A receptor antagonists. J Med Chem52:4955-9 (2009) [PubMed] Article