| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Amine oxidase [flavin-containing] B |
|---|
| Ligand | BDBM50249871 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_619728 (CHEMBL1111192) |
|---|
| IC50 | 32810±n/a nM |
|---|
| Citation |  Chimenti, F; Carradori, S; Secci, D; Bolasco, A; Bizzarri, B; Chimenti, P; Granese, A; Yáñez, M; Orallo, F Synthesis and inhibitory activity against human monoamine oxidase of N1-thiocarbamoyl-3,5-di(hetero)aryl-4,5-dihydro-(1H)-pyrazole derivatives. Eur J Med Chem45:800-4 (2010) [PubMed] Article Chimenti, F; Carradori, S; Secci, D; Bolasco, A; Bizzarri, B; Chimenti, P; Granese, A; Yáñez, M; Orallo, F Synthesis and inhibitory activity against human monoamine oxidase of N1-thiocarbamoyl-3,5-di(hetero)aryl-4,5-dihydro-(1H)-pyrazole derivatives. Eur J Med Chem45:800-4 (2010) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Amine oxidase [flavin-containing] B |
|---|
| Name: | Amine oxidase [flavin-containing] B |
|---|
| Synonyms: | AOFB_HUMAN | MAO-B | MAOB | Monoamine oxidase type B | Monoamine oxidase type B (MAO B) | Monoamine oxidase type B (MAO B) | Monoamine oxidase type B (MAOB) |
|---|
| Type: | Protein |
|---|
| Mol. Mass.: | 58768.76 |
|---|
| Organism: | Homo sapiens (Human) |
|---|
| Description: | P27338 |
|---|
| Residue: | 520 |
|---|
| Sequence: | MSNKCDVVVVGGGISGMAAAKLLHDSGLNVVVLEARDRVGGRTYTLRNQKVKYVDLGGSY
VGPTQNRILRLAKELGLETYKVNEVERLIHHVKGKSYPFRGPFPPVWNPITYLDHNNFWR
TMDDMGREIPSDAPWKAPLAEEWDNMTMKELLDKLCWTESAKQLATLFVNLCVTAETHEV
SALWFLWYVKQCGGTTRIISTTNGGQERKFVGGSGQVSERIMDLLGDRVKLERPVIYIDQ
TRENVLVETLNHEMYEAKYVISAIPPTLGMKIHFNPPLPMMRNQMITRVPLGSVIKCIVY
YKEPFWRKKDYCGTMIIDGEEAPVAYTLDDTKPEGNYAAIMGFILAHKARKLARLTKEER
LKKLCELYAKVLGSLEALEPVHYEEKNWCEEQYSGGCYTTYFPPGILTQYGRVLRQPVDR
IYFAGTETATHWSGYMEGAVEAGERAAREILHAMGKIPEDEIWQSEPESVDVPAQPITTT
FLERHLPSVPGLLRLIGLTTIFSATALGFLAHKRGLLVRV
|
|
|
|---|
| BDBM50249871 |
|---|
| n/a |
|---|
| Name | BDBM50249871 |
|---|
| Synonyms: | 3-phenyl-5-p-tolyl-4,5-dihydro-1H-pyrazole-1-carbothioamide | Amino[5-(4-methylphenyl)-3-phenyl(2-pyrazolinyl)]methane-1-thione | CHEMBL489743 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C17H17N3S |
|---|
| Mol. Mass. | 295.402 |
|---|
| SMILES | Cc1ccc(cc1)C1CC(=NN1C(N)=S)c1ccccc1 |c:10| |
|---|
| Structure | 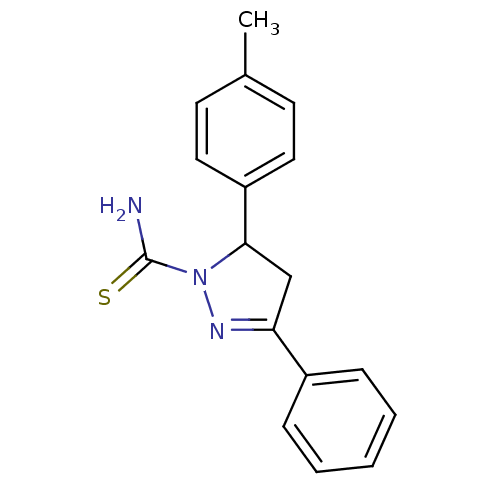
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Chimenti, F; Carradori, S; Secci, D; Bolasco, A; Bizzarri, B; Chimenti, P; Granese, A; Yáñez, M; Orallo, F Synthesis and inhibitory activity against human monoamine oxidase of N1-thiocarbamoyl-3,5-di(hetero)aryl-4,5-dihydro-(1H)-pyrazole derivatives. Eur J Med Chem45:800-4 (2010) [PubMed] Article
Chimenti, F; Carradori, S; Secci, D; Bolasco, A; Bizzarri, B; Chimenti, P; Granese, A; Yáñez, M; Orallo, F Synthesis and inhibitory activity against human monoamine oxidase of N1-thiocarbamoyl-3,5-di(hetero)aryl-4,5-dihydro-(1H)-pyrazole derivatives. Eur J Med Chem45:800-4 (2010) [PubMed] Article