| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform |
|---|
| Ligand | BDBM35578 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_628563 (CHEMBL1104685) |
|---|
| IC50 | 141±n/a nM |
|---|
| Citation |  Verheijen, JC; Richard, DJ; Curran, K; Kaplan, J; Lefever, M; Nowak, P; Malwitz, DJ; Brooijmans, N; Toral-Barza, L; Zhang, WG; Lucas, J; Hollander, I; Ayral-Kaloustian, S; Mansour, TS; Yu, K; Zask, A Discovery of 4-morpholino-6-aryl-1H-pyrazolo[3,4-d]pyrimidines as highly potent and selective ATP-competitive inhibitors of the mammalian target of rapamycin (mTOR): optimization of the 6-aryl substituent. J Med Chem52:8010-24 (2009) [PubMed] Article Verheijen, JC; Richard, DJ; Curran, K; Kaplan, J; Lefever, M; Nowak, P; Malwitz, DJ; Brooijmans, N; Toral-Barza, L; Zhang, WG; Lucas, J; Hollander, I; Ayral-Kaloustian, S; Mansour, TS; Yu, K; Zask, A Discovery of 4-morpholino-6-aryl-1H-pyrazolo[3,4-d]pyrimidines as highly potent and selective ATP-competitive inhibitors of the mammalian target of rapamycin (mTOR): optimization of the 6-aryl substituent. J Med Chem52:8010-24 (2009) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform |
|---|
| Name: | Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform |
|---|
| Synonyms: | PI3-kinase p110 subunit beta | PI3-kinase subunit p110-beta | PI3Kbeta | PIK3C1 | PIK3CB | PK3CB_HUMAN | Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta (PI3Kbeta) | Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform (PI3K beta) | Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform (PI3K) | Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform (PI3K-beta) | Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform (PI3Kbeta) | Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform (PI3Kÿ²) | Phosphatidylinositol-4,5-bisphosphate 3-kinase catalytic subunit beta isoform | Phosphoinositide 3-Kinase (PI3K), beta | Phosphoinositide 3-Kinase (PI3K), beta Chain A | Phosphoinositide-3-kinase (PI3K beta) | PtdIns-3-kinase p110 |
|---|
| Type: | Enzyme Subunit |
|---|
| Mol. Mass.: | 122769.00 |
|---|
| Organism: | Homo sapiens (Human) |
|---|
| Description: | P42338 |
|---|
| Residue: | 1070 |
|---|
| Sequence: | MCFSFIMPPAMADILDIWAVDSQIASDGSIPVDFLLPTGIYIQLEVPREATISYIKQMLW
KQVHNYPMFNLLMDIDSYMFACVNQTAVYEELEDETRRLCDVRPFLPVLKLVTRSCDPGE
KLDSKIGVLIGKGLHEFDSLKDPEVNEFRRKMRKFSEEKILSLVGLSWMDWLKQTYPPEH
EPSIPENLEDKLYGGKLIVAVHFENCQDVFSFQVSPNMNPIKVNELAIQKRLTIHGKEDE
VSPYDYVLQVSGRVEYVFGDHPLIQFQYIRNCVMNRALPHFILVECCKIKKMYEQEMIAI
EAAINRNSSNLPLPLPPKKTRIISHVWENNNPFQIVLVKGNKLNTEETVKVHVRAGLFHG
TELLCKTIVSSEVSGKNDHIWNEPLEFDINICDLPRMARLCFAVYAVLDKVKTKKSTKTI
NPSKYQTIRKAGKVHYPVAWVNTMVFDFKGQLRTGDIILHSWSSFPDELEEMLNPMGTVQ
TNPYTENATALHVKFPENKKQPYYYPPFDKIIEKAAEIASSDSANVSSRGGKKFLPVLKE
ILDRDPLSQLCENEMDLIWTLRQDCREIFPQSLPKLLLSIKWNKLEDVAQLQALLQIWPK
LPPREALELLDFNYPDQYVREYAVGCLRQMSDEELSQYLLQLVQVLKYEPFLDCALSRFL
LERALGNRRIGQFLFWHLRSEVHIPAVSVQFGVILEAYCRGSVGHMKVLSKQVEALNKLK
TLNSLIKLNAVKLNRAKGKEAMHTCLKQSAYREALSDLQSPLNPCVILSELYVEKCKYMD
SKMKPLWLVYNNKVFGEDSVGVIFKNGDDLRQDMLTLQMLRLMDLLWKEAGLDLRMLPYG
CLATGDRSGLIEVVSTSETIADIQLNSSNVAAAAAFNKDALLNWLKEYNSGDDLDRAIEE
FTLSCAGYCVASYVLGIGDRHSDNIMVKKTGQLFHIDFGHILGNFKSKFGIKRERVPFIL
TYDFIHVIQQGKTGNTEKFGRFRQCCEDAYLILRRHGNLFITLFALMLTAGLPELTSVKD
IQYLKDSLALGKSEEEALKQFKQKFDEALRESWTTKVNWMAHTVRKDYRS
|
|
|
|---|
| BDBM35578 |
|---|
| n/a |
|---|
| Name | BDBM35578 |
|---|
| Synonyms: | pyrazolo pyrimidine, 10 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C28H33N9O2 |
|---|
| Mol. Mass. | 527.6207 |
|---|
| SMILES | CNC(=O)Nc1ccc(cc1)-c1nc(N2CCOCC2)c2cnn(C3CCN(Cc4cccnc4)CC3)c2n1 |
|---|
| Structure | 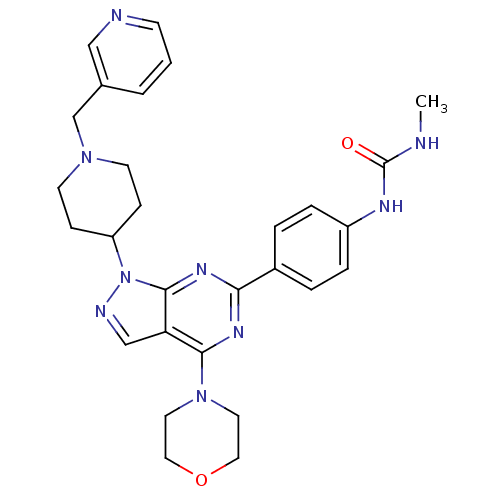
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Verheijen, JC; Richard, DJ; Curran, K; Kaplan, J; Lefever, M; Nowak, P; Malwitz, DJ; Brooijmans, N; Toral-Barza, L; Zhang, WG; Lucas, J; Hollander, I; Ayral-Kaloustian, S; Mansour, TS; Yu, K; Zask, A Discovery of 4-morpholino-6-aryl-1H-pyrazolo[3,4-d]pyrimidines as highly potent and selective ATP-competitive inhibitors of the mammalian target of rapamycin (mTOR): optimization of the 6-aryl substituent. J Med Chem52:8010-24 (2009) [PubMed] Article
Verheijen, JC; Richard, DJ; Curran, K; Kaplan, J; Lefever, M; Nowak, P; Malwitz, DJ; Brooijmans, N; Toral-Barza, L; Zhang, WG; Lucas, J; Hollander, I; Ayral-Kaloustian, S; Mansour, TS; Yu, K; Zask, A Discovery of 4-morpholino-6-aryl-1H-pyrazolo[3,4-d]pyrimidines as highly potent and selective ATP-competitive inhibitors of the mammalian target of rapamycin (mTOR): optimization of the 6-aryl substituent. J Med Chem52:8010-24 (2009) [PubMed] Article