

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Reaction Details | |||
|---|---|---|---|
 | Report a problem with these data | ||
| Target | Urotensin-2 receptor | ||
| Ligand | BDBM50320472 | ||
| Substrate/Competitor | n/a | ||
| Meas. Tech. | ChEMBL_636746 (CHEMBL1167035) | ||
| EC50 | 0.34±n/a nM | ||
| Citation |  Maryanoff, BE; Kinney, WA Urotensin-II receptor modulators as potential drugs. J Med Chem53:2695-708 (2010) [PubMed] Article Maryanoff, BE; Kinney, WA Urotensin-II receptor modulators as potential drugs. J Med Chem53:2695-708 (2010) [PubMed] Article | ||
| More Info.: | Get all data from this article, Assay Method | ||
| Urotensin-2 receptor | |||
| Name: | Urotensin-2 receptor | ||
| Synonyms: | G-protein coupled receptor 14 | G-protein coupled sensory epithelial neuropeptide-like receptor | Gpr14 | Senr | UR-II-R | UR2R_RAT | Urotensin-II | Uts2r | ||
| Type: | Enzyme Catalytic Domain | ||
| Mol. Mass.: | 42725.34 | ||
| Organism: | RAT | ||
| Description: | Urotensin-II UTS2R RAT::P49684 | ||
| Residue: | 386 | ||
| Sequence: |
| ||
| BDBM50320472 | |||
| n/a | |||
| Name | BDBM50320472 | ||
| Synonyms: | (3S,6S,9S,15S)-3-((4R,7S,10S,13S,16S,19R)-13-((1H-indol-3-yl)methyl)-10-(4-aminobutyl)-16-benzyl-4-((S)-1-carboxy-2-methylpropylcarbamoyl)-7-(naphthalen-2-ylmethyl)-6,9,12,15,18-pentaoxo-1,2-dithia-5,8,11,14,17-pentaazacycloicosan-19-ylcarbamoyl)-15-amino-9-((R)-1-hydroxyethyl)-6-methyl-5,8,11,14-tetraoxo-4,7,10,13-tetraazahexadecan-1-oic acid | CHEMBL1165797 | ||
| Type | Small organic molecule | ||
| Emp. Form. | C66H86N14O16S2 | ||
| Mol. Mass. | 1395.603 | ||
| SMILES | CC(C)[C@H](NC(=O)[C@@H]1CSSC[C@H](NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](C)NC(=O)[C@@H](NC(=O)CNC(=O)[C@H](C)N)[C@@H](C)O)C(=O)N[C@@H](Cc2ccccc2)C(=O)N[C@@H](Cc2c[nH]c3ccccc23)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](Cc2ccc3ccccc3c2)C(=O)N1)C(O)=O |r| | ||
| Structure | 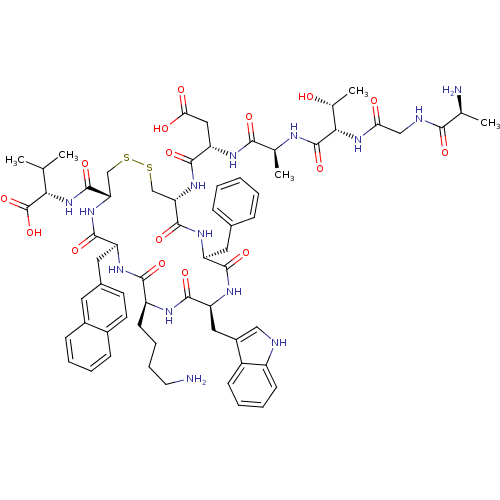
| ||